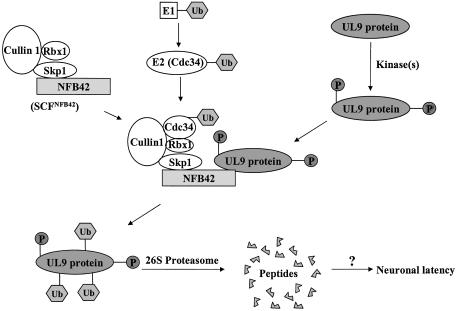
Fig. 4.
Model depicting the role of NFB42 in the recognition and ubiquitination of HSV-1 UL9 protein. The UL9 protein is phosphorylated in HSV-1-infected cells by cellular kinase(s). In neurons, the phosphorylated UL9 protein can be recognized by NFB42, a neuron-specific F-box protein. NFB42 is linked to the SCF complex by interaction between the F box within NFB42 and Skp1, which results in the formation of SCFNFB42. Skp1 also binds to cullin-1; Cdc34, an E2 ubiquitin-conjugating enzyme, binds to the SCFNFB42 and ubiquitinates the UL9 protein bound to NFB42. Rbx1 functions in E2 recruitment. Cullin-1 functions as a bridging molecule in the SCF complex. The polyubiquitinated UL9 proteins are degraded by the 26S proteasome, leading to the inhibition of initiation of HSV-1 DNA replication and, ultimately, to neuronal HSV-1 latency.