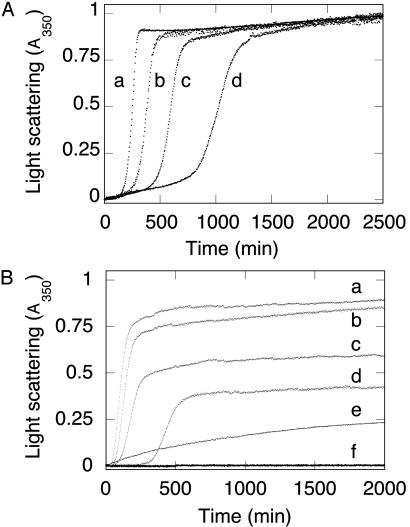
Fig. 2.
Aggregation kinetics of wild-type FBP28 WW domain as a function of peptide concentration. (A) Light scattering of wild-type FBP28 WW versus peptide concentration at 37°C. Traces: a, 500 μM FBP28; b, 200 μM FBP28; c, 100 μM FBP28; d, 50 μM FBP28. Data were normalized to a common scale by dividing the observed absorbance at a given time by the signal at the end of the experiment. At all concentrations >10 μM, the reactions reached an apparent endpoint, with >95% of the peptide having been precipitated by centrifugation after an ≈24-h incubation. (B) Comparison of aggregation kinetics of wild-type and mutant FBP28 WW domains. Peptide (100 μM) at 37°C: Traces: a, wild-type FBP28, ionic strength = 150 mM; b, wild type, ionic strength = 100 mM; c, wild type, ionic strength = 50 mM; d, wild type, ionic strength = 16 mM; e, FBP28 W30A, ionic strength = 150 mM; f, oxidized FBP28 V5C/E35C, ionic strength = 150 mM. Under conditions where wild-type FBP28 aggregation is faster than at lower ionic strength, we saw no aggregation for FBP28 V5C/E35C. Identical results were obtained for the disulfide-reduced mutant. Lower-order association is observed for FBP28 W30A, which is consistent with the 10-nm particles seen by using electron microscopy.