Figure 2.
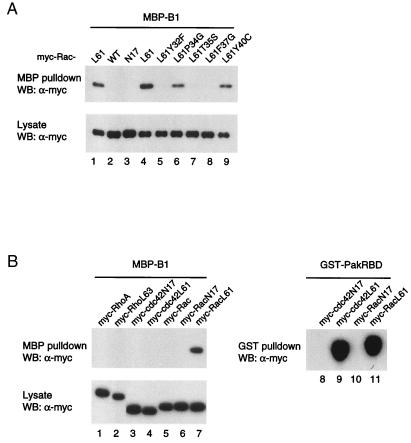
(A) Mutations in the Rac effector domain abolish plexin-B1 interaction. Various myc-Rac mutants were transfected into HEK293 cells, and cell lysates were subjected to in vitro pulldown by MBP-plexin-B1. Expression of different Rac mutants in cell lysates is shown (lower). The mutations Y32F (lane 5), T35S (lane7), and F37L (lane 9) in the Rac effector domain completely abolished interaction with plexin-B1. In contrast, the mutations P34G (lane 6) and Y40C (lane 9) had no significant effect on the interaction with plexin-B1. (B) Cdc42 and RhoA do not interact with plexin-B1. Various mutants of myc-RhoA, -Cdc42, and -Rac were transfected into HEK293 cells. Cell lysates were used in either MBP-plexin-B1 or GST-PAK-RBD pulldown. Neither RhoL63 (lane 2) nor Cdc42L61 (lane 4) bind to plexin-B1. As a control, GST-PAK-RBD binds both RacL61 (lane 9) and Cdc42L61 (lane 11).