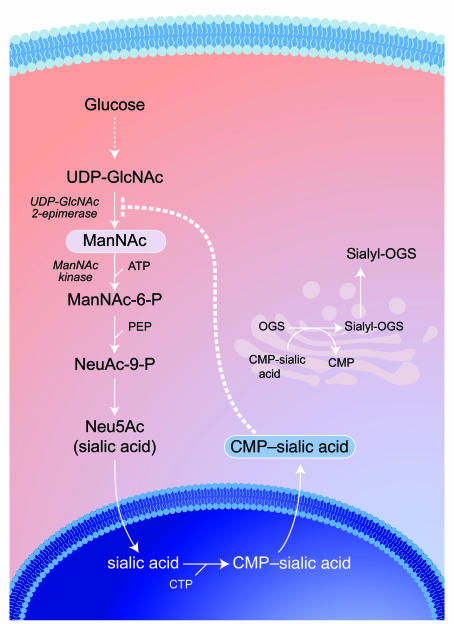
Figure 1. Intracellular sialic acid metabolism.
Cytosolic glucose is converted in several steps into UDP-GlcNAc, which serves as substrate for the bifunctional, rate-limiting, committed enzyme of sialic acid biosynthesis, GNE/MNK. The GNE catalytic activity (EC 5.1.3.14) epimerizes UDP-GlcNAc to ManNAc, followed by the phosphorylation of ManNAc to ManNAc-6-phosphate (MaNAc-6-P) by the MNK kinase catalytic domain (EC 2.7.1.60). ManNAc-6-P is then further converted into Neu5Ac (sialic acid), which is activated into cytidine monophosphate–sialic acid (CMP–sialic acid) in the nucleus. CMP–sialic acid can subsequently be utilized in the Golgi complex as a substrate for the biosynthesis of sialyl-oligosaccharides by sialyltransferases. Cytosolic CMP–sialic acid displays strong feedback inhibition (dotted line) of GNE enzymatic activity by binding to its allosteric site (1), thereby contributing to the tight regulation of intracellular sialic acid biosynthesis. Even more complexity is added to this pathway by the presence of ancillary kinases, such as GlcNAc kinase (NAGK; EC 2.7.1.59) with high intrinsic MNK activity (19), which can also convert ManNAc to ManNAc-6-P. CTP, cytidine triphosphate; PEP, phosphoenolpyruvate; OGS, oligosaccharides.