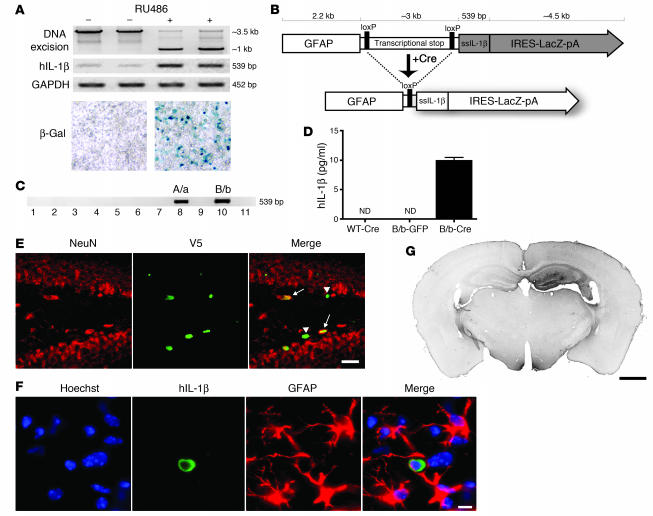
Figure 1. Engineering and testing of the IL-1βXAT mouse.
(A) The 293GLVP/CrePr stable cell line was transiently transfected with CMV/IL-1βXAT and cultured with or without RU486. RU486 caused DNA excision and expression of hIL-1β and β-galactosidase by RT-PCR and X-gal histochemistry, respectively. (B) The linear IL-1βXAT construct (~10 kb) consisted of a murine GFAP promoter; a transcriptional stop element flanked by loxP sequences; downstream ssIL-1β cDNA (11); and an internal ribosome entry site (IRES) followed by the β-gal coding sequence (LacZ) and bovine growth hormone polyadenylation signal (pA). Exposure to Cre recombinase caused excision of the transcriptional stop and subsequent transcriptional activation of ssIL-1β. (C) PCR screening of the 11 live-born IL-1βXAT pups using ssIL-1β–specific primers. Transgenic founder lines A/a and B/b are shown. (D) ELISA quantification (mean ± SEM) of hIL-1β protein supernatant concentration in individual primary astrocyte (n = 4) cultures from B/b and WT astrocytes transduced with FIV-Cre or FIV-GFP. ND, not detected (i.e., below detection limits). (E) The epicenter of viral transduction, the dentate gyrus, was bounded by NeuN-positive neurons (red stain). Colocalization of the epitope tag V5 (green stain) expressed by FIV-Cre demonstrated transduction of both neuronal (arrows) and non-neuronal cells (arrowheads). (F) Demonstration of hIL-1β expression (green stain) by astrocytes (red stain) in the dentate gyrus. Hoechst (blue stain) labeled cell nuclei. (G) MHC class II–stained coronal section from a B/b animal 1 week after intrahippocampal FIV-Cre injection (right hemisphere). Scale bars: 25 μm (E); 10 μm (F); 1 μm (G).