Abstract
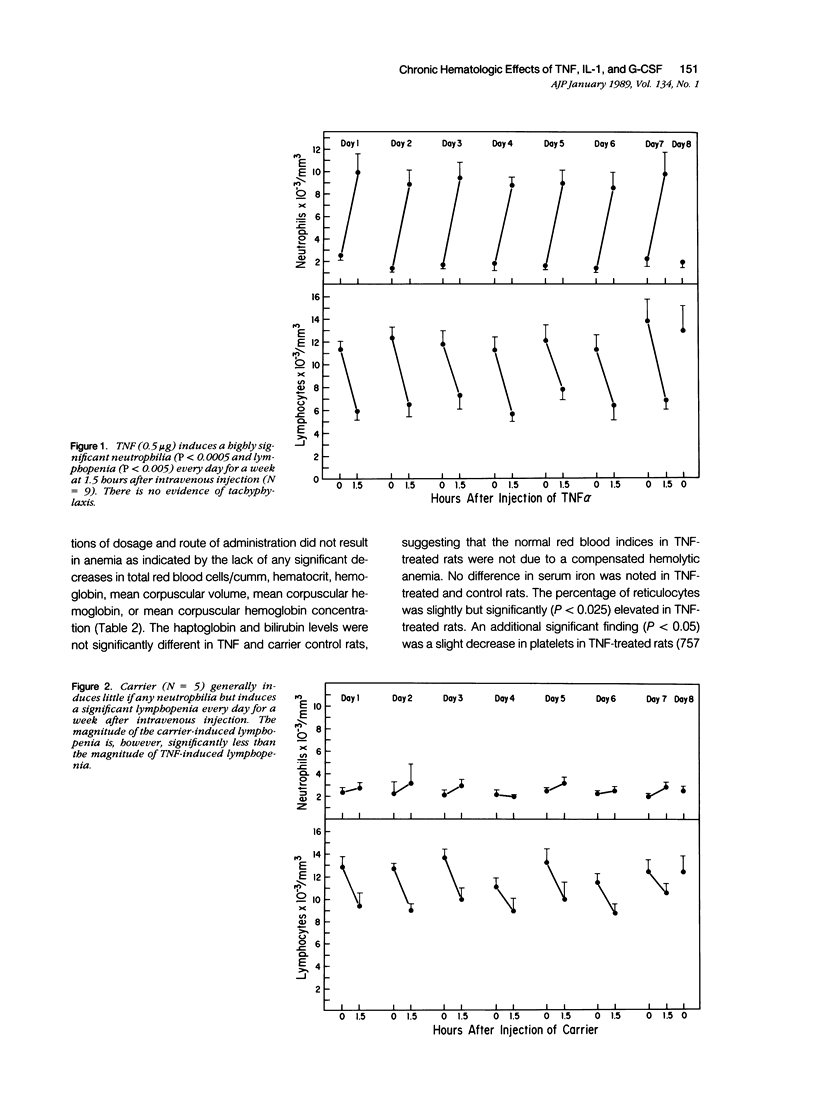
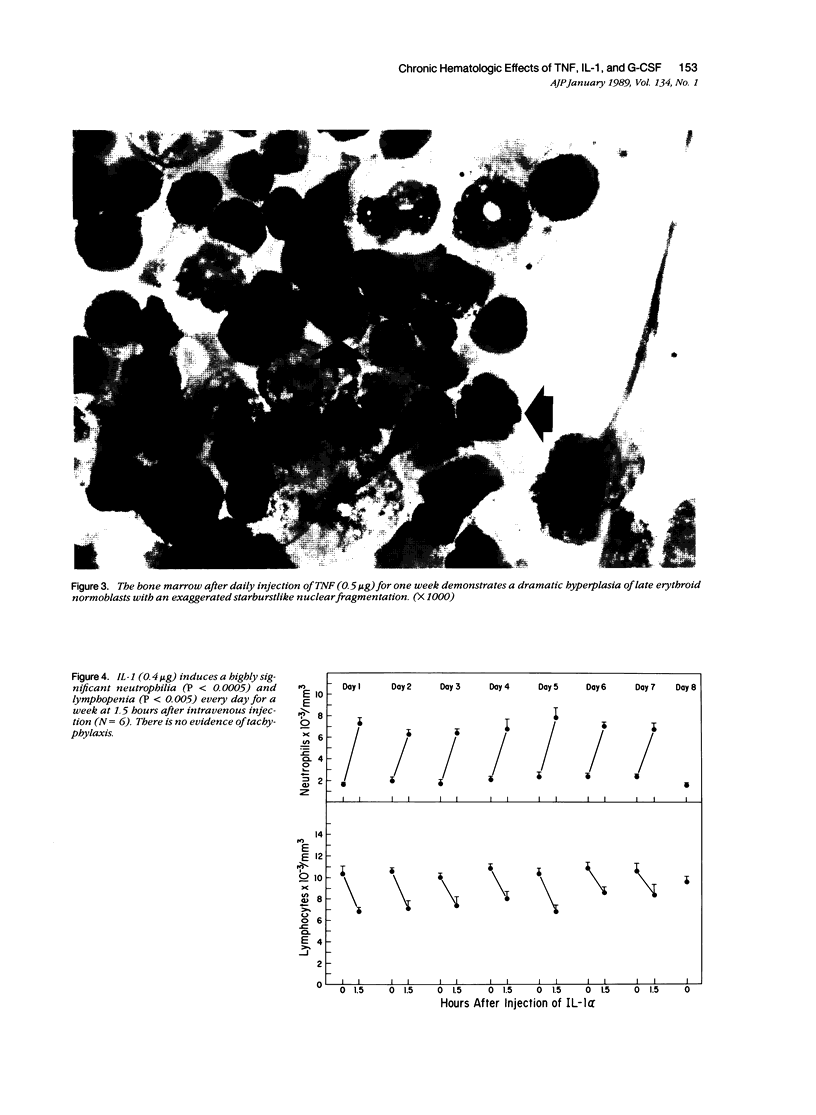
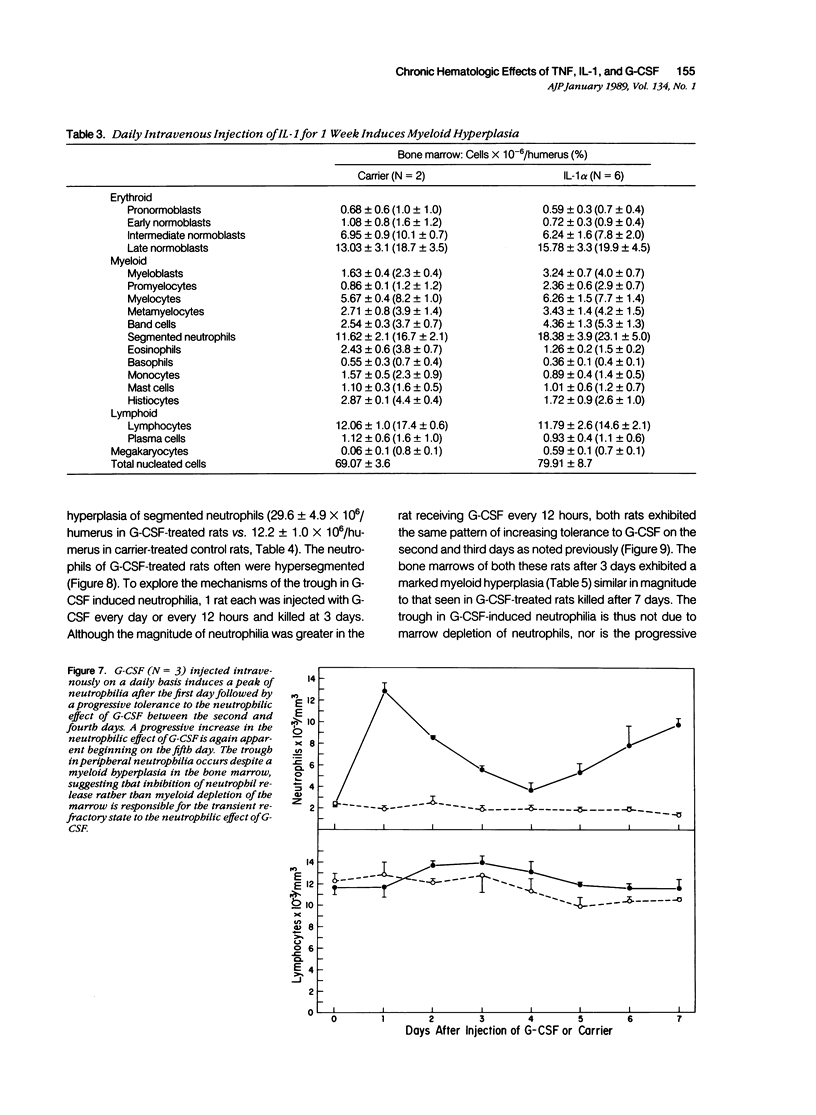
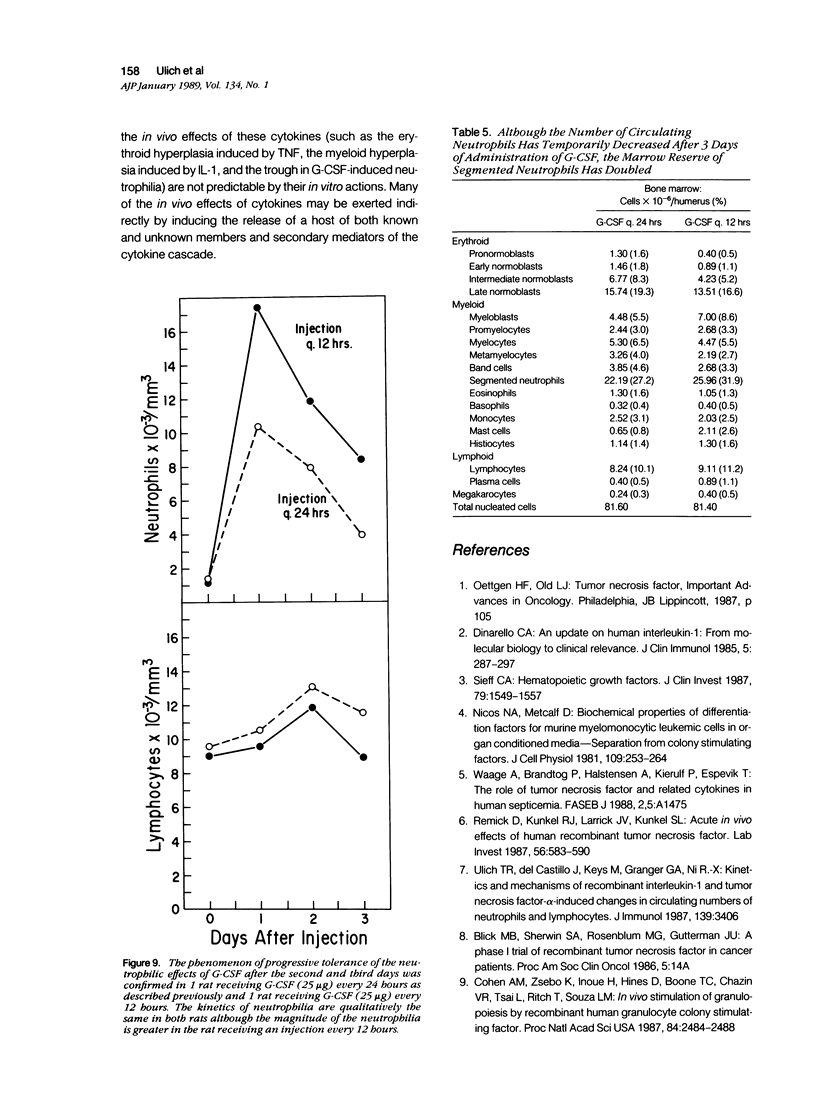
Monokines may contribute to the regulation of hematopoiesis and circulating numbers of leukocytes during chronic inflammation. The hematologic effects of daily intravenous injection of the recombinant monokines tumor necrosis factor (TNF), interleukin-1 (IL-1), and granulocyte-colony stimulating factor (G-CSF) were therefore studied in the bone marrow and circulation of rats over the course of a week. TNF induced daily neutrophilia and lymphopenia with no evidence of tachyphylaxis. TNF also induced a slight decrease in early myeloid forms in the marrow, but, more strikingly, induced a marked erythroid hyperplasia of late normoblasts, although no changes other than a slight reticulocytosis were noted in the peripheral red blood cell compartment. IL-1 also induced daily neutrophilia and lymphopenia with no evidence of tachyphylaxis. IL-1 differed from TNF in the induction of a significant myeloid hyperplasia and in the lack of any effect on the erythroid elements of the marrow. The lack of tachyphylaxis to the chronic administration of both TNF and IL-1 suggests that the mechanism of endotoxin-induced tachyphylaxis is not at the level of the effector cell response to these endogenous cytokines. G-CSF induced a biphasic peripheral neutrophilia first peaking on day one, reaching a nadir on day 4, and then rising progressively again until day 7. The low level of neutrophilia on day 4 is not due to marrow depletion of neutrophils secondary to the neutrophil releasing activity of G-CSF because the marrows of G-CSF-treated rats on both days 3 and 7 contained over twice the number of mature neutrophils as controls. Thus, the trough in the neutrophilia induced by G-CSF is postulated to be due to an as-yet unidentified negative feedback mechanism that inhibits neutrophil release from the marrow.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagby G. C., Jr, Dinarello C. A., Wallace P., Wagner C., Hefeneider S., McCall E. Interleukin 1 stimulates granulocyte macrophage colony-stimulating activity release by vascular endothelial cells. J Clin Invest. 1986 Nov;78(5):1316–1323. doi: 10.1172/JCI112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Williams D. E., Lu L., Cooper S., Anderson S. L., Beyer G. S., Hoffman R., Rubin B. Y. The suppressive influences of human tumor necrosis factors on bone marrow hematopoietic progenitor cells from normal donors and patients with leukemia: synergism of tumor necrosis factor and interferon-gamma. J Immunol. 1986 Jun 15;136(12):4487–4495. [PubMed] [Google Scholar]
- Burchett S. K., Weaver W. M., Westall J. A., Larsen A., Kronheim S., Wilson C. B. Regulation of tumor necrosis factor/cachectin and IL-1 secretion in human mononuclear phagocytes. J Immunol. 1988 May 15;140(10):3473–3481. [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R. Granulocytic hyperplasia and induction of tolerance in response to chronic endotoxin administration. J Reticuloendothel Soc. 1971 Mar;9(3):288–297. [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R., Marsh J. C., Cartwright G. E., Wintrobe M. M. Quantitative studies of blood and bone marrow neutrophils in normal mice. Am J Physiol. 1968 Aug;215(2):353–360. doi: 10.1152/ajplegacy.1968.215.2.353. [DOI] [PubMed] [Google Scholar]
- Cohen A. M., Zsebo K. M., Inoue H., Hines D., Boone T. C., Chazin V. R., Tsai L., Ritch T., Souza L. M. In vivo stimulation of granulopoiesis by recombinant human granulocyte colony-stimulating factor. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2484–2488. doi: 10.1073/pnas.84.8.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz I. G., Movat H. Z. Desensitization of acute inflammatory lesions to chemotaxins and endotoxin. J Immunol. 1984 Oct;133(4):2163–2168. [PubMed] [Google Scholar]
- Cybulsky M. I., Chan M. K., Movat H. Z. Acute inflammation and microthrombosis induced by endotoxin, interleukin-1, and tumor necrosis factor and their implication in gram-negative infection. Lab Invest. 1988 Apr;58(4):365–378. [PubMed] [Google Scholar]
- Cybulsky M. I., Cybulsky I. J., Movat H. Z. Neutropenic responses to intradermal injections of Escherichia coli. Effects on the kinetics of polymorphonuclear leukocyte emigration. Am J Pathol. 1986 Jul;124(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. An update on human interleukin-1: from molecular biology to clinical relevance. J Clin Immunol. 1985 Sep;5(5):287–297. doi: 10.1007/BF00918247. [DOI] [PubMed] [Google Scholar]
- Greisman S. E., Young E. J., Workman J. B., Ollodart R. M., Hornick R. B. Mechanisms of endotoxin tolerance. The role of the spleen. J Clin Invest. 1975 Dec;56(6):1597–1607. doi: 10.1172/JCI108242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULSE E. V. QUANTITATIVE CELL COUNTS OF THE BONE MARROW AND BLOOD AND THEIR SECULAR VARIATIONS IN THE NORMAL ADULT RAT. Acta Haematol. 1964 Jan;31:50–63. doi: 10.1159/000209613. [DOI] [PubMed] [Google Scholar]
- Hemmi H., Nakamura T., Tamura K., Shimizu Y., Kato S., Miki T., Takahashi N., Muramatsu M., Numao N., Sugamura K. Lymphotoxin: induction of terminal differentiation of the human myeloid leukemia cell lines HL-60 and THP-1. J Immunol. 1987 Feb 1;138(3):664–666. [PubMed] [Google Scholar]
- Koeffler H. P., Gasson J., Ranyard J., Souza L., Shepard M., Munker R. Recombinant human TNF alpha stimulates production of granulocyte colony-stimulating factor. Blood. 1987 Jul;70(1):55–59. [PubMed] [Google Scholar]
- Moore M. A., Warren D. J. Synergy of interleukin 1 and granulocyte colony-stimulating factor: in vivo stimulation of stem-cell recovery and hematopoietic regeneration following 5-fluorouracil treatment of mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7134–7138. doi: 10.1073/pnas.84.20.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase T., Hotta T., Ohno R., Saito H. Predominant suppression of neutrophil colony growth by recombinant human tumor necrosis factor. Proc Soc Exp Biol Med. 1987 Nov;186(2):188–191. doi: 10.3181/00379727-186-42601. [DOI] [PubMed] [Google Scholar]
- Murase T., Hotta T., Saito H., Ohno R. Effect of recombinant human tumor necrosis factor on the colony growth of human leukemia progenitor cells and normal hematopoietic progenitor cells. Blood. 1987 Feb;69(2):467–472. [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Biochemical properties of differentiation factors for murine myelomonocytic leukemic cells in organ conditioned media--separation from colony-stimulating factors. J Cell Physiol. 1981 Nov;109(2):253–264. doi: 10.1002/jcp.1041090208. [DOI] [PubMed] [Google Scholar]
- Remick D. G., Kunkel R. G., Larrick J. W., Kunkel S. L. Acute in vivo effects of human recombinant tumor necrosis factor. Lab Invest. 1987 Jun;56(6):583–590. [PubMed] [Google Scholar]
- Sieff C. A. Hematopoietic growth factors. J Clin Invest. 1987 Jun;79(6):1549–1557. doi: 10.1172/JCI112988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., del Castillo J., Keys M., Granger G. A., Ni R. X. Kinetics and mechanisms of recombinant human interleukin 1 and tumor necrosis factor-alpha-induced changes in circulating numbers of neutrophils and lymphocytes. J Immunol. 1987 Nov 15;139(10):3406–3415. [PubMed] [Google Scholar]
- Ulich T. R., del Castillo J., Souza L. Kinetics and mechanisms of recombinant human granulocyte-colony stimulating factor-induced neutrophilia. Am J Pathol. 1988 Dec;133(3):630–638. [PMC free article] [PubMed] [Google Scholar]
- Welte K., Bonilla M. A., Gillio A. P., Boone T. C., Potter G. K., Gabrilove J. L., Moore M. A., O'Reilly R. J., Souza L. M. Recombinant human granulocyte colony-stimulating factor. Effects on hematopoiesis in normal and cyclophosphamide-treated primates. J Exp Med. 1987 Apr 1;165(4):941–948. doi: 10.1084/jem.165.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]