Abstract
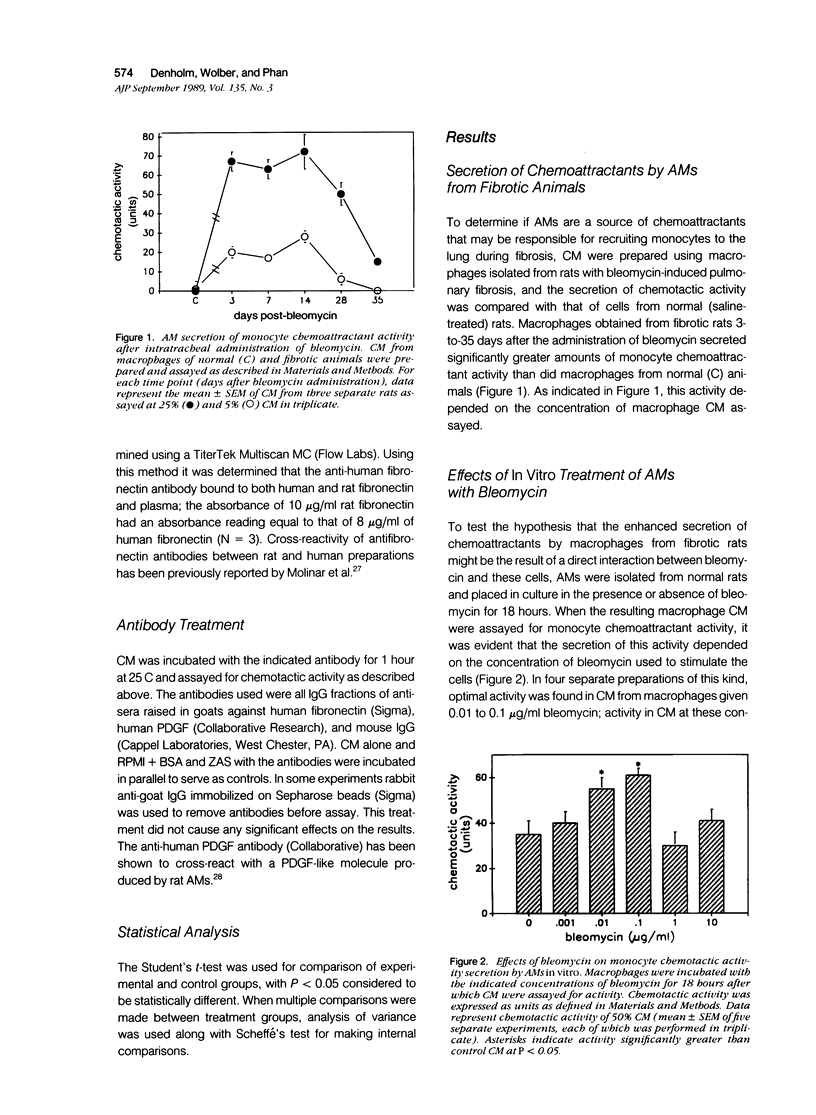
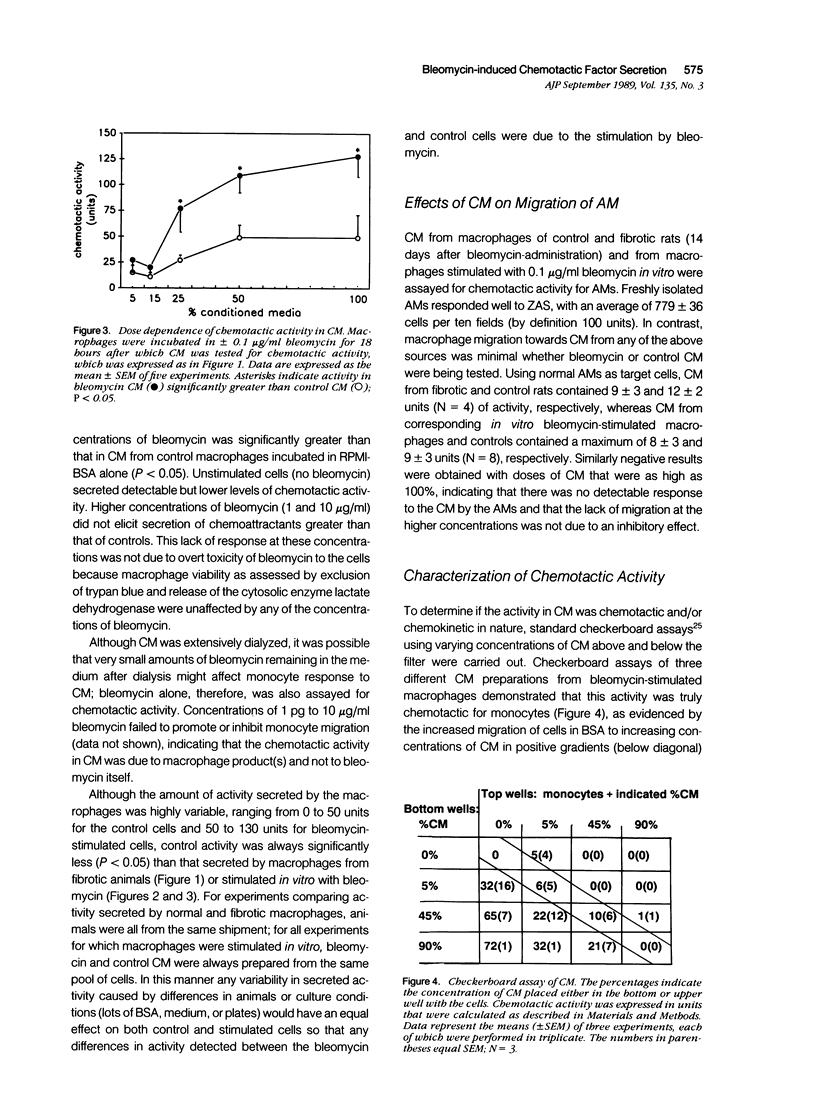
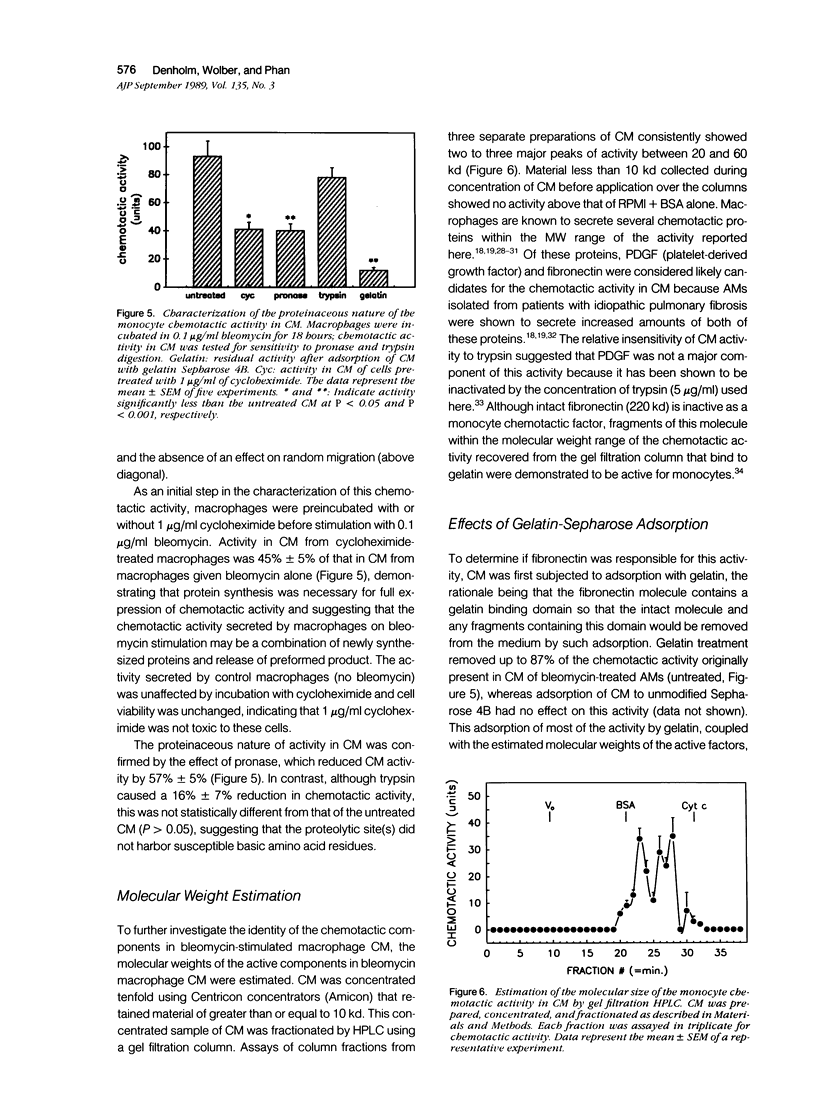
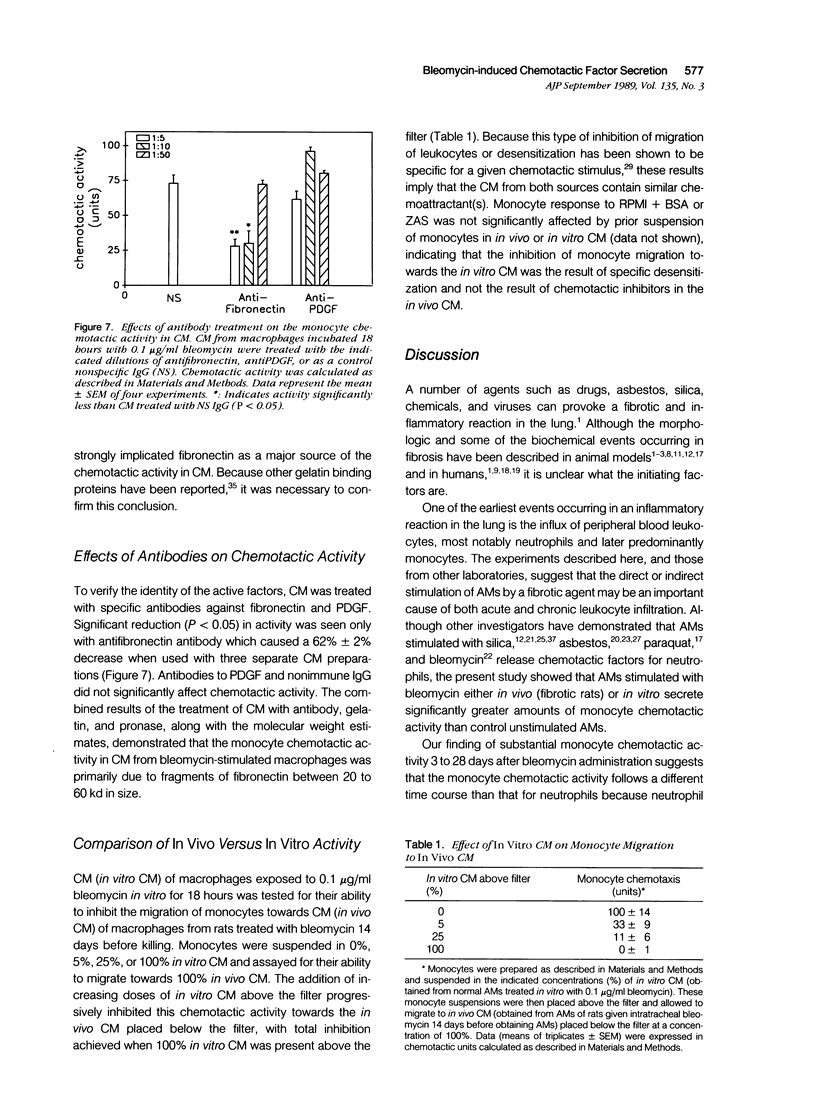
The purpose of this study was to determine if alveolar macrophages (AMs) are a source of monocyte chemoattractants and the role bleomycin interaction with AMs may play in the recruitment of monocytes to the lung in a rodent model of bleomycin-induced pulmonary fibrosis. AMs isolated from rats with bleomycin-induced fibrosis secreted significantly greater amounts of monocyte chemoattractants than those isolated from normal rats. When AMs from normal rats were stimulated with bleomycin in vitro, monocyte chemotactic activity was secreted into the medium. Chemotactic activity secretion by AM stimulated with 0.01 to 0.1 micrograms/ml bleomycin was significantly higher than that of cells incubated in medium alone. This activity was truly chemotactic for monocytes, but caused only minimal migration of normal AMs. Bleomycin itself at concentrations of 1 pg/ml to 10 micrograms/ml had no monocyte chemoattractant activity. Characterization of the chemotactic activity in conditioned media (CM) from bleomycin-stimulated AM demonstrated that the major portion of the activity bound to gelatin, was heterogeneous, with estimated molecular weights of 20 to 60 kd, and was inactivated by specific antifibronectin antibody. These findings suggest that fibronectin fragments are primarily responsible for the monocyte chemotactic activity secreted by AMs. Through increased secretion of such chemotactic substances, AMs could play a key role in the recruitment of peripheral blood monocytes into the lung in inflammatory lung disease and fibrosis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Role of monocytes and interstitial cells in the generation of alveolar macrophages II. Kinetic studies after carbon loading. Lab Invest. 1980 May;42(5):518–524. [PubMed] [Google Scholar]
- Bitterman P. B., Saltzman L. E., Adelberg S., Ferrans V. J., Crystal R. G. Alveolar macrophage replication. One mechanism for the expansion of the mononuclear phagocyte population in the chronically inflamed lung. J Clin Invest. 1984 Aug;74(2):460–469. doi: 10.1172/JCI111443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blussé van Oud Alblas A., Mattie H., van Furth R. A quantitative evaluation of pulmonary macrophage kinetics. Cell Tissue Kinet. 1983 May;16(3):211–219. [PubMed] [Google Scholar]
- Blussé van Oud Alblas A., van der Linden-Schrever B., Van Furth R. Origin and kinetics of pulmonary macrophages during an inflammatory reaction induced by intra-alveolar administration of aerosolized heat-killed BCG. Am Rev Respir Dis. 1983 Aug;128(2):276–281. doi: 10.1164/arrd.1983.128.2.276. [DOI] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Adaptive responses of the pulmonary macrophagic system to carbon. I. Kinetic studies. Lab Invest. 1978 Apr;38(4):422–429. [PubMed] [Google Scholar]
- Chandler D. B., Hyde D. M., Giri S. N. Morphometric estimates of infiltrative cellular changes during the development of bleomycin-induced pulmonary fibrosis in hamsters. Am J Pathol. 1983 Aug;112(2):170–177. [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Wikner N. E., Doherty D. E., Norris D. A. Cryptic chemotactic activity of fibronectin for human monocytes resides in the 120-kDa fibroblastic cell-binding fragment. J Biol Chem. 1988 Aug 25;263(24):12115–12123. [PubMed] [Google Scholar]
- Click E. M., Balian G. Domain structure of human plasma and cellular fibronectin. Use of a monoclonal antibody and heparin affinity to identify three different subunit chains. Biochemistry. 1985 Nov 5;24(23):6685–6696. doi: 10.1021/bi00344a058. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. The macrophage--versatile element of inflammation. Harvey Lect. 1981 1982;77:63–80. [PubMed] [Google Scholar]
- Denholm E. M., Lewis J. C. Monocyte chemoattractants in pigeon aortic atherosclerosis. Am J Pathol. 1987 Mar;126(3):464–475. [PMC free article] [PubMed] [Google Scholar]
- Denholm E. M., Phan S. H. The effects of bleomycin on alveolar macrophage growth factor secretion. Am J Pathol. 1989 Feb;134(2):355–363. [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Falk W., Leonard E. J. Specificity and reversibility of chemotactic deactivation of human monocytes. Infect Immun. 1981 May;32(2):464–468. doi: 10.1128/iai.32.2.464-468.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava R. A., McClure D. B. Fibronectin-associated transforming growth factor. J Cell Physiol. 1987 May;131(2):184–189. doi: 10.1002/jcp.1041310207. [DOI] [PubMed] [Google Scholar]
- Frolik C. A., Dart L. L., Meyers C. A., Smith D. M., Sporn M. B. Purification and initial characterization of a type beta transforming growth factor from human placenta. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3676–3680. doi: 10.1073/pnas.80.12.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek J. E., Kelman J. A., Fells G., Weinberger S. E., Horwitz A. L., Reynolds H. Y., Fulmer J. D., Crystal R. G. Collagenase in the lower respiratory tract of patients with idiopathic pulmonary fibrosis. N Engl J Med. 1979 Oct 4;301(14):737–742. doi: 10.1056/NEJM197910043011401. [DOI] [PubMed] [Google Scholar]
- Glassroth J. L., Bernardo J., Lucey E. C., Center D. M., Jung-Legg Y. J., Snider G. L. Interstitial pulmonary fibrosis induced in hamsters by intratracheally administered chrysotile asbestos. Histology, lung mechanics, and inflammatory events. Am Rev Respir Dis. 1984 Aug;130(2):242–248. doi: 10.1164/arrd.1984.130.2.242. [DOI] [PubMed] [Google Scholar]
- Kaelin R. M., Center D. M., Bernardo J., Grant M., Snider G. L. The role of macrophage-derived chemoattractant activities in the early inflammatory events of bleomycin-induced pulmonary injury. Am Rev Respir Dis. 1983 Jul;128(1):132–137. doi: 10.1164/arrd.1983.128.1.132. [DOI] [PubMed] [Google Scholar]
- Kagan E., Oghiso Y., Hartmann D. P. Enhanced release of a chemoattractant for alveolar macrophages after asbestos inhalation. Am Rev Respir Dis. 1983 Oct;128(4):680–687. doi: 10.1164/arrd.1983.128.4.680. [DOI] [PubMed] [Google Scholar]
- Kumar R. K., Bennett R. A., Brody A. R. A homologue of platelet-derived growth factor produced by rat alveolar macrophages. FASEB J. 1988 Apr;2(7):2272–2277. doi: 10.1096/fasebj.2.7.3280379. [DOI] [PubMed] [Google Scholar]
- Lazo J. S., Pham E. T. Pulmonary fate of [3H]bleomycin A2 in mice. J Pharmacol Exp Ther. 1984 Jan;228(1):13–18. [PubMed] [Google Scholar]
- Lemaire I., Beaudoin H., Massé S., Grondin C. Alveolar macrophage stimulation of lung fibroblast growth in asbestos-induced pulmonary fibrosis. Am J Pathol. 1986 Feb;122(2):205–211. [PMC free article] [PubMed] [Google Scholar]
- Lugano E. M., Dauber J. H., Daniele R. P. Acute experimental silicosis. Lung morphology, histology, and macrophage chemotaxin secretion. Am J Pathol. 1982 Oct;109(1):27–36. [PMC free article] [PubMed] [Google Scholar]
- Lugano E. M., Dauber J. H., Daniele R. P. Silica stimulation of chemotactic factor release by guinea pig alveolar macrophages. J Reticuloendothel Soc. 1981 Nov;30(5):381–390. [PubMed] [Google Scholar]
- Martin T. R., Ayars G., Butler J., Altman L. C. The comparative toxicity of volcanic ash and quartz. Effects on cells derived from the human lung. Am Rev Respir Dis. 1984 Nov;130(5):778–782. doi: 10.1164/arrd.1984.130.5.778. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Kelley D. G. Degradation of fibronectin by human leukocyte elastase. Release of biologically active fragments. J Biol Chem. 1980 Sep 25;255(18):8848–8858. [PubMed] [Google Scholar]
- Mensing H., Czarnetzki B. M. Leukotriene B4 induces in vitro fibroblast chemotaxis. J Invest Dermatol. 1984 Jan;82(1):9–12. doi: 10.1111/1523-1747.ep12258678. [DOI] [PubMed] [Google Scholar]
- Miller K., Calverley A., Kagan E. Evidence of a quartz-induced chemotactic factor for guinea pig alveolar macrophages. Environ Res. 1980 Jun;22(1):31–39. doi: 10.1016/0013-9351(80)90116-4. [DOI] [PubMed] [Google Scholar]
- Molnar J., Gelder F. B., Lai M. Z., Siefring G. E., Jr, Credo R. B., Lorand L. Purification of opsonically active human and rat cold-insoluble globulin (plasma fibronectin). Biochemistry. 1979 Sep 4;18(18):3909–3916. doi: 10.1021/bi00585a010. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Yamamoto T., Ishimatsu T., Kambara T. A high-molecular-weight trypsinlike protease in the skin sites of delayed hypersensitivity in guinea pigs. Am J Pathol. 1984 Feb;114(2):250–263. [PMC free article] [PubMed] [Google Scholar]
- Norris D. A., Clark R. A., Swigart L. M., Huff J. C., Weston W. L., Howell S. E. Fibronectin fragment(s) are chemotactic for human peripheral blood monocytes. J Immunol. 1982 Oct;129(4):1612–1618. [PubMed] [Google Scholar]
- Phan S. H., Kunkel S. L. Inhibition of bleomycin-induced pulmonary fibrosis by nordihydroguaiaretic acid. The role of alveolar macrophage activation and mediator production. Am J Pathol. 1986 Aug;124(2):343–352. [PMC free article] [PubMed] [Google Scholar]
- Phan S. H., McGarry B. M., Loeffler K. M., Kunkel S. L. Binding of leukotriene C4 to rat lung fibroblasts and stimulation of collagen synthesis in vitro. Biochemistry. 1988 Apr 19;27(8):2846–2853. doi: 10.1021/bi00408a028. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Hayman E. G., Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981 Oct;26(2 Pt 2):259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Crystal R. G. Fibronectin in human bronchopulmonary lavage fluid. Elevation in patients with interstitial lung disease. J Clin Invest. 1982 Jan;69(1):113–122. doi: 10.1172/JCI110421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Hunninghake G. W., Bitterman P. B., Crystal R. G. Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7147–7151. doi: 10.1073/pnas.78.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberger C. I., Hunninghake G. W., Kawanami O., Ferrans V. J., Crystal R. G. Role of alveolar macrophages in asbestosis: modulation of neutrophil migration to the lung after acute asbestos exposure. Thorax. 1982 Nov;37(11):803–809. doi: 10.1136/thx.37.11.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberger C. I., Rennard S. I., Bitterman P. B., Fukuda Y., Ferrans V. J., Crystal R. G. Paraquat-induced pulmonary fibrosis. Role of the alveolitis in modulating the development of fibrosis. Am Rev Respir Dis. 1984 Jan;129(1):168–173. doi: 10.1164/arrd.1984.129.1.168. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Huang J. S., Griffin G. L., Deuel T. F. Dissociation of the chemotactic and mitogenic activities of platelet-derived growth factor by human neutrophil elastase. J Cell Biol. 1985 Feb;100(2):351–356. doi: 10.1083/jcb.100.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellito J., Esparza C., Armstrong C. Maintenance of the normal rat alveolar macrophage cell population. The roles of monocyte influx and alveolar macrophage proliferation in situ. Am Rev Respir Dis. 1987 Jan;135(1):78–82. doi: 10.1164/arrd.1987.135.1.78. [DOI] [PubMed] [Google Scholar]
- Spurzem J. R., Saltini C., Rom W., Winchester R. J., Crystal R. G. Mechanisms of macrophage accumulation in the lungs of asbestos-exposed subjects. Am Rev Respir Dis. 1987 Aug;136(2):276–280. doi: 10.1164/ajrccm/136.2.276. [DOI] [PubMed] [Google Scholar]
- Thrall R. S., Barton R. W., D'Amato D. A., Sulavik S. B. Differential cellular analysis of bronchoalveolar lavage fluid obtained at various stages during the development of bleomycin-induced pulmonary fibrosis in the rat. Am Rev Respir Dis. 1982 Sep;126(3):488–492. doi: 10.1164/arrd.1982.126.3.488. [DOI] [PubMed] [Google Scholar]
- Villiger B., Kelley D. G., Engleman W., Kuhn C., 3rd, McDonald J. A. Human alveolar macrophage fibronectin: synthesis, secretion, and ultrastructural localization during gelatin-coated latex particle binding. J Cell Biol. 1981 Sep;90(3):711–720. doi: 10.1083/jcb.90.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. M., Colella S., Allavena P., Mantovani A. Chemotactic activity of human recombinant granulocyte-macrophage colony-stimulating factor. Immunology. 1987 Mar;60(3):439–444. [PMC free article] [PubMed] [Google Scholar]
- Williams H. R., Lin T. Y. Human polymorphonuclear leukocyte collagenase and gelatinase. Comparison of certain enzymatic properties. Int J Biochem. 1984;16(12):1321–1329. doi: 10.1016/0020-711x(84)90235-0. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Martinet Y., Crystal R. G. Modulation of fibronectin gene expression in human mononuclear phagocytes. J Clin Invest. 1987 Dec;80(6):1720–1727. doi: 10.1172/JCI113263. [DOI] [PMC free article] [PubMed] [Google Scholar]


