Abstract
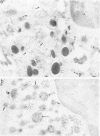
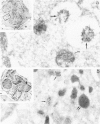
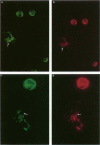
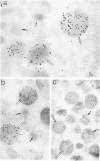
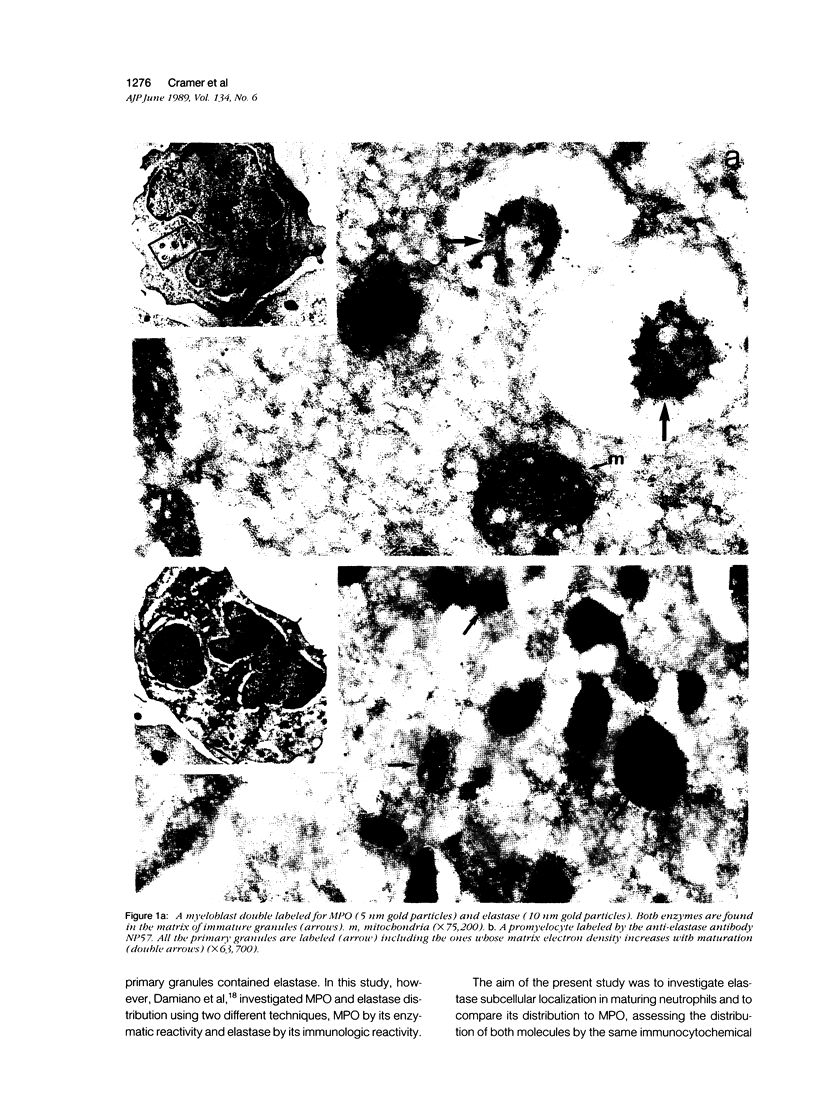
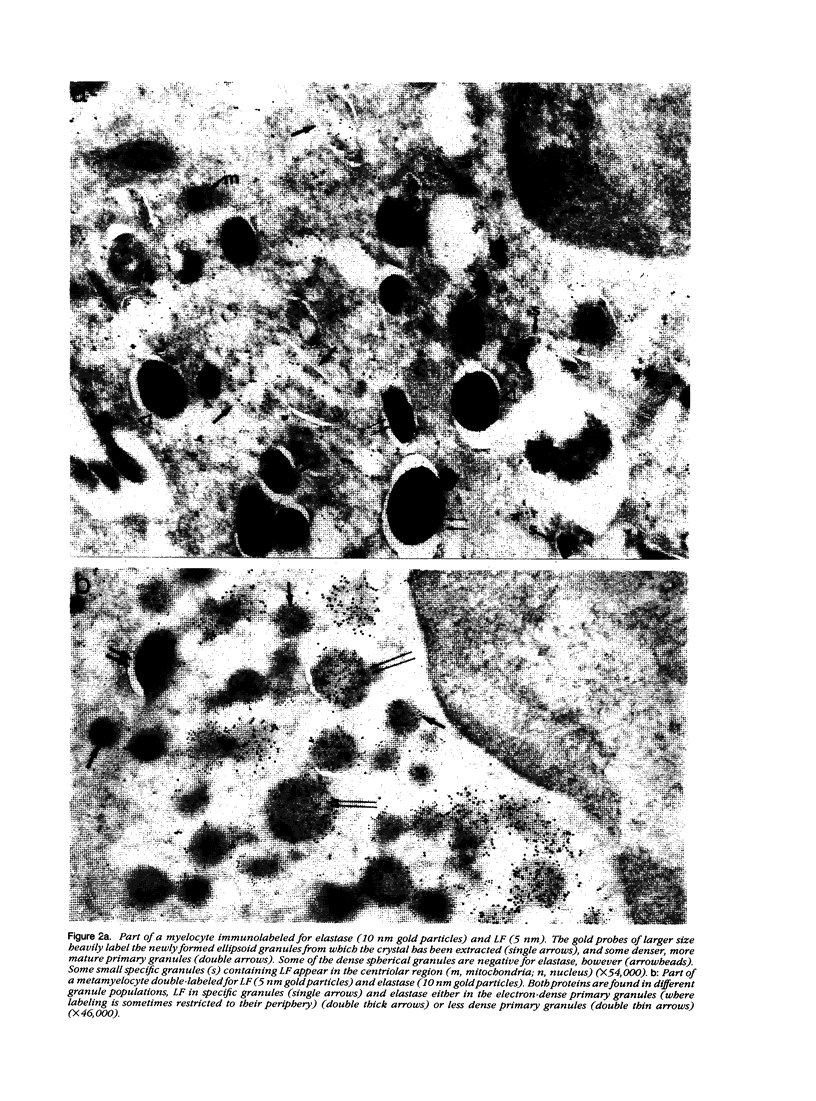
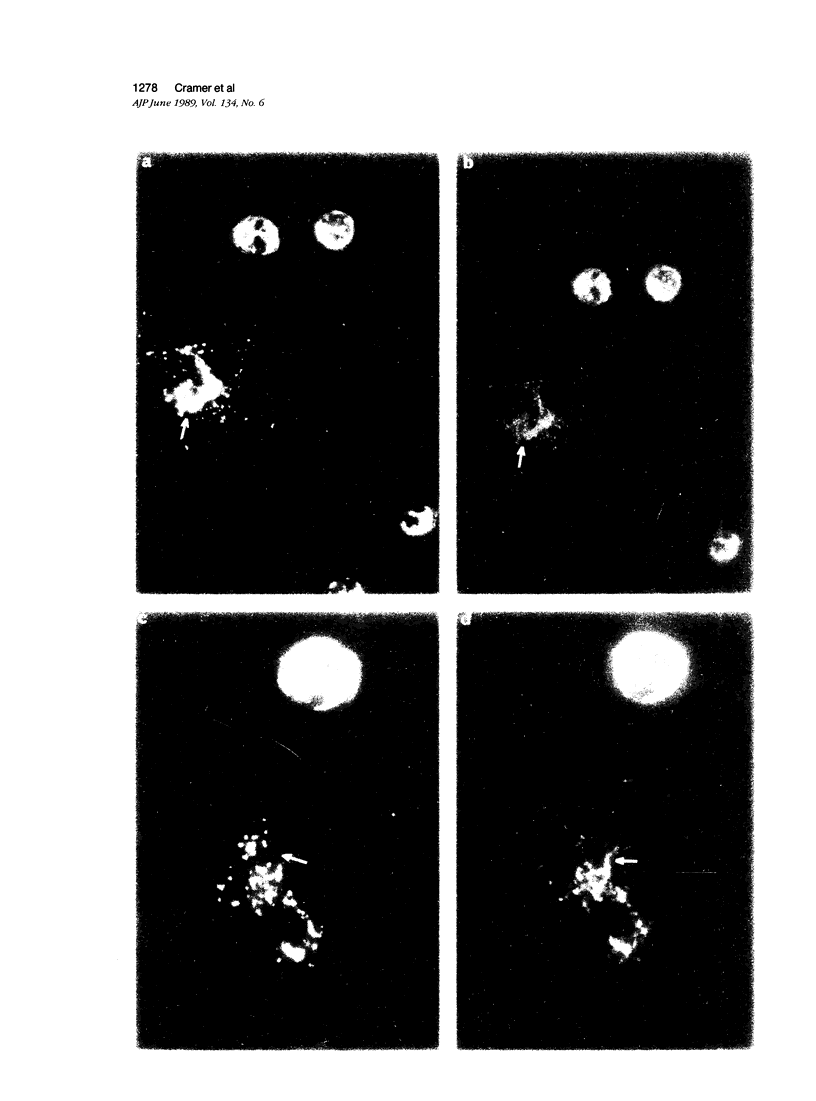
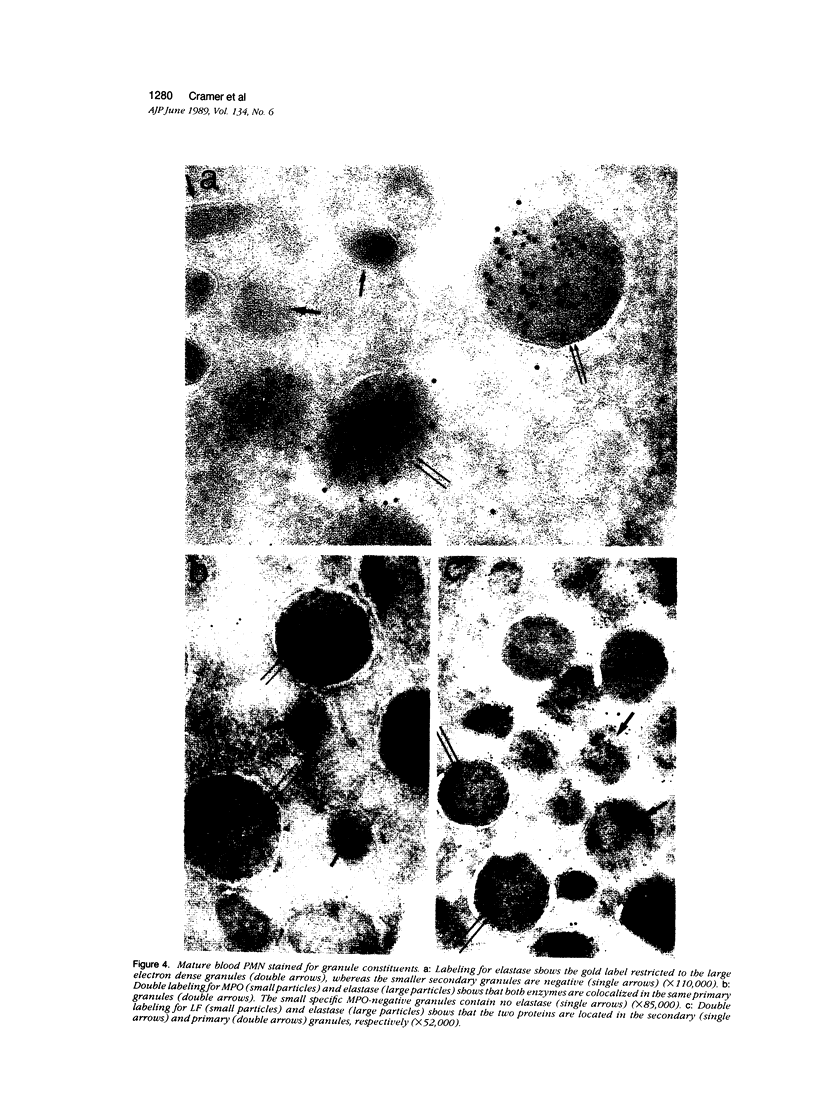
The authors have localized elastase in human blood and bone marrow neutrophils by immunoelectron microscopy using a monoclonal anti-human elastase antibody (NP 57) and compared its distribution with myeloperoxidase (MPO) and lactoferrin (LF), which mark primary and secondary neutrophil granule, respectively. Human bone marrow and blood polymorphonuclear leukocytes (PMN), either unstimulated or after phagocytosis of latex microbeads, were fixed in 4% paraformaldehyde. Ultrathin frozen sections were immunolabeled with NP 57, followed by an immunogold probe. In bone marrow granulocyte precursors elastase appeared simultaneously in the immature first granules of myeloblasts with MPO. As these granules became denser with maturation, labeling for both enzymes became weaker and sometimes negative (possibly due to masking of immunoreactivity). The ellipsoidal primary granules were strongly labeled by NP57. LF positive granules appeared later, at the myelocyte stage, and contained neither MPO nor elastase. In mature neutrophils, immunolabeling for elastase was found together with MPO in the large electron-dense primary granules and in a different granule population from the LF-positive secondary granules. Double labeling with two different-sized gold particles was used to compare the kinetics of degranulation of secondary and primary granules. The observation and the analysis of single phagosome content was made possible by this new technique. In conclusion, immunoelectron microscopy was used to show elastase in the primary granules of neutrophils, where it appears simultaneously with MPO. This technique has also allowed comparison of the kinetics of degranulation of both types of granules, and could be applied to different experimental and pathologic conditions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton D. F. Sequential degranulation of the two types of polymorphonuclear leukocyte granules during phagocytosis of microorganisms. J Cell Biol. 1973 Aug;58(2):249–264. doi: 10.1083/jcb.58.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton-Gorius J., Reyes F. Ultrastructure of human bone marrow cell maturation. Int Rev Cytol. 1976;46:251–321. doi: 10.1016/s0074-7696(08)60993-6. [DOI] [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Clark J. M., Vaughan D. W., Aiken B. M., Kagan H. M. Elastase-like enzymes in human neutrophils localized by ultrastructural cytochemistry. J Cell Biol. 1980 Jan;84(1):102–119. doi: 10.1083/jcb.84.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer E. M., Breton-Gorius J. Ultrastructural localization of lysozyme in human neutrophils by immunogold. J Leukoc Biol. 1987 Mar;41(3):242–247. doi: 10.1002/jlb.41.3.242. [DOI] [PubMed] [Google Scholar]
- Cramer E., Auclair C., Hakim J., Feliu E., Boucherot J., Troube H., Bernard J. F., Bergogne E., Boivin P. Metabolic activity of phagocytosing granulocytes in chronic granulocytic leukemia: ultrastructural observation of a degranulation defect. Blood. 1977 Jul;50(1):93–106. [PubMed] [Google Scholar]
- Cramer E., Pryzwansky K. B., Villeval J. L., Testa U., Breton-Gorius J. Ultrastructural localization of lactoferrin and myeloperoxidase in human neutrophils by immunogold. Blood. 1985 Feb;65(2):423–432. [PubMed] [Google Scholar]
- Crocker J., Jenkins R., Burnett D. Immunohistochemical demonstration of leucocyte elastase in human tissues. J Clin Pathol. 1984 Oct;37(10):1114–1118. doi: 10.1136/jcp.37.10.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano V. V., Kucich U., Murer E., Laudenslager N., Weinbaum G. Ultrastructural quantitation of peroxidase- and elastase-containing granules in human neutrophils. Am J Pathol. 1988 May;131(2):235–245. [PMC free article] [PubMed] [Google Scholar]
- Damiano V. V., Tsang A., Kucich U., Abrams W. R., Rosenbloom J., Kimbel P., Fallahnejad M., Weinbaum G. Immunolocalization of elastase in human emphysematous lungs. J Clin Invest. 1986 Aug;78(2):482–493. doi: 10.1172/JCI112600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Bretz U., Baggiolini M. Release of gelatinase from a novel secretory compartment of human neutrophils. J Clin Invest. 1982 Sep;70(3):518–525. doi: 10.1172/JCI110643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R. C., Peterson C. G., Segal A. W., Venge P. Elastase in the different primary granules of the human neutrophil. Biochem Biophys Res Commun. 1985 Nov 15;132(3):1130–1136. doi: 10.1016/0006-291x(85)91924-2. [DOI] [PubMed] [Google Scholar]
- Janoff A. Elastase in tissue injury. Annu Rev Med. 1985;36:207–216. doi: 10.1146/annurev.me.36.020185.001231. [DOI] [PubMed] [Google Scholar]
- Kinkade J. M., Jr, Pember S. O., Barnes K. C., Shapira R., Spitznagel J. K., Martin L. E. Differential distribution of distinct forms of myeloperoxidase in different azurophilic granule subpopulations from human neutrophils. Biochem Biophys Res Commun. 1983 Jul 18;114(1):296–303. doi: 10.1016/0006-291x(83)91627-3. [DOI] [PubMed] [Google Scholar]
- Kramps J. A., van der Valk P., van der Sandt M. M., Lindeman J., Meijer C. J. Elastase as a marker for neutrophilic myeloid cells. J Histochem Cytochem. 1984 Apr;32(4):389–394. doi: 10.1177/32.4.6561228. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of granulocyte elastases. Eur J Biochem. 1974 Mar 1;42(2):519–527. doi: 10.1111/j.1432-1033.1974.tb03367.x. [DOI] [PubMed] [Google Scholar]
- Perianin A., Labro M. T., Hakim J. Chemokinetic activity of N-formyl-methionyl-leucyl-phenylalanine on human neutrophils, and its modulation by phenylbutazone. Biochem Pharmacol. 1982 Oct 1;31(19):3071–3076. doi: 10.1016/0006-2952(82)90082-x. [DOI] [PubMed] [Google Scholar]
- Pryzwansky K. B., Breton-Gorius J. Identification of a subpopulation of primary granules in human neutrophils based upon maturation and distribution. Study by transmission electron microscopy cytochemistry and high voltage electron microscopy of whole cell preparations. Lab Invest. 1985 Dec;53(6):664–671. [PubMed] [Google Scholar]
- Pryzwansky K. B., Martin L. E., Spitznagel J. K. Immunocytochemical localization of myeloperoxidase, lactoferrin, lysozyme and neutral proteases in human monocytes and neutrophilic granulocytes. J Reticuloendothel Soc. 1978 Sep;24(3):295–310. [PubMed] [Google Scholar]
- Pryzwansky K. B., Rausch P. G., Spitznagel J. K., Herion J. C. Immunocytochemical distinction between primary and secondary granule formation in developing human neutrophils: correlations with Romanowsky stains. Blood. 1979 Feb;53(2):179–185. [PubMed] [Google Scholar]
- Pulford K. A., Erber W. N., Crick J. A., Olsson I., Micklem K. J., Gatter K. C., Mason D. Y. Use of monoclonal antibody against human neutrophil elastase in normal and leukaemic myeloid cells. J Clin Pathol. 1988 Aug;41(8):853–860. doi: 10.1136/jcp.41.8.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W. G., Ganz T., Kinkade J. M., Jr, Selsted M. E., Lehrer R. I., Parmley R. T. Defensin-rich dense granules of human neutrophils. Blood. 1987 Sep;70(3):757–765. [PubMed] [Google Scholar]
- Ruth R. C., Weglicki W. B. Mechanisms of loss of latency of lysosomal enzymes. Effects of incubation on the properties of lysosomal membranes. Biochem J. 1980 Jan 15;186(1):243–256. doi: 10.1042/bj1860243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Dorling J., Coade S. Kinetics of fusion of the cytoplasmic granules with phagocytic vacuoles in human polymorphonuclear leukocytes. Biochemical and morphological studies. J Cell Biol. 1980 Apr;85(1):42–59. doi: 10.1083/jcb.85.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Campbell E. J. Neutral proteinases from human inflammatory cells. A critical review of their role in extracellular matrix degradation. Clin Lab Med. 1983 Dec;3(4):645–666. [PubMed] [Google Scholar]
- Spitznagel J. K., Dalldorf F. G., Leffell M. S., Folds J. D., Welsh I. R., Cooney M. H., Martin L. E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest. 1974 Jun;30(6):774–785. [PubMed] [Google Scholar]
- Tapia F. J., Varndell I. M., Probert L., De Mey J., Polak J. M. Double immunogold staining method for the simultaneous ultrastructural localization of regulatory peptides. J Histochem Cytochem. 1983 Jul;31(7):977–981. doi: 10.1177/31.7.6189888. [DOI] [PubMed] [Google Scholar]