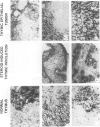
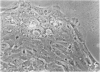
Abstract
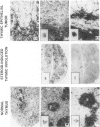
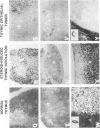
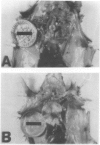
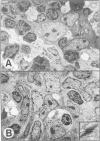
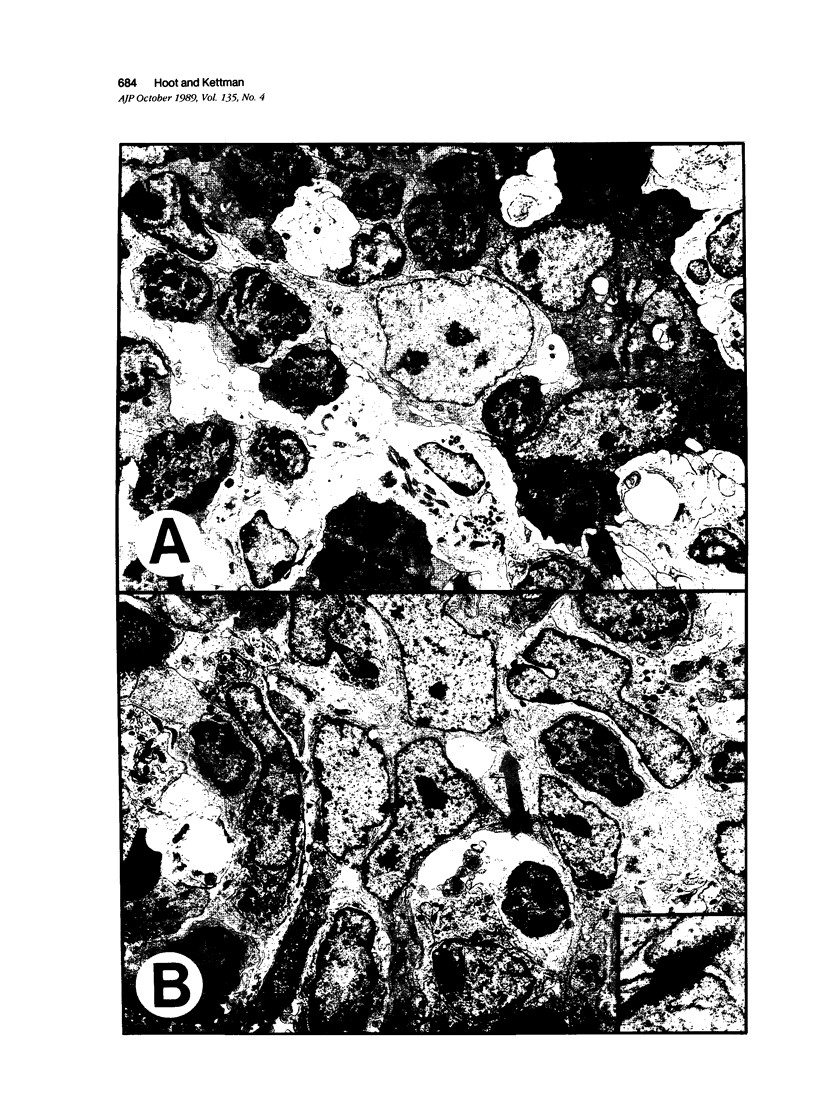
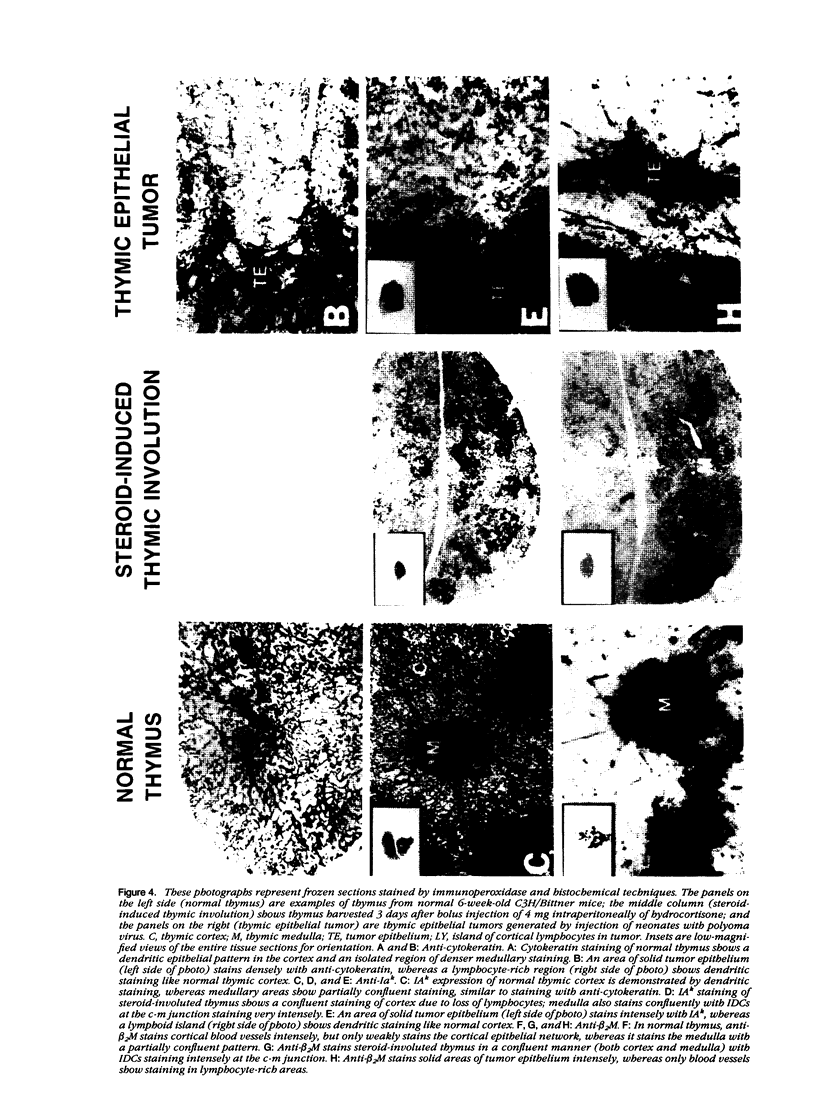
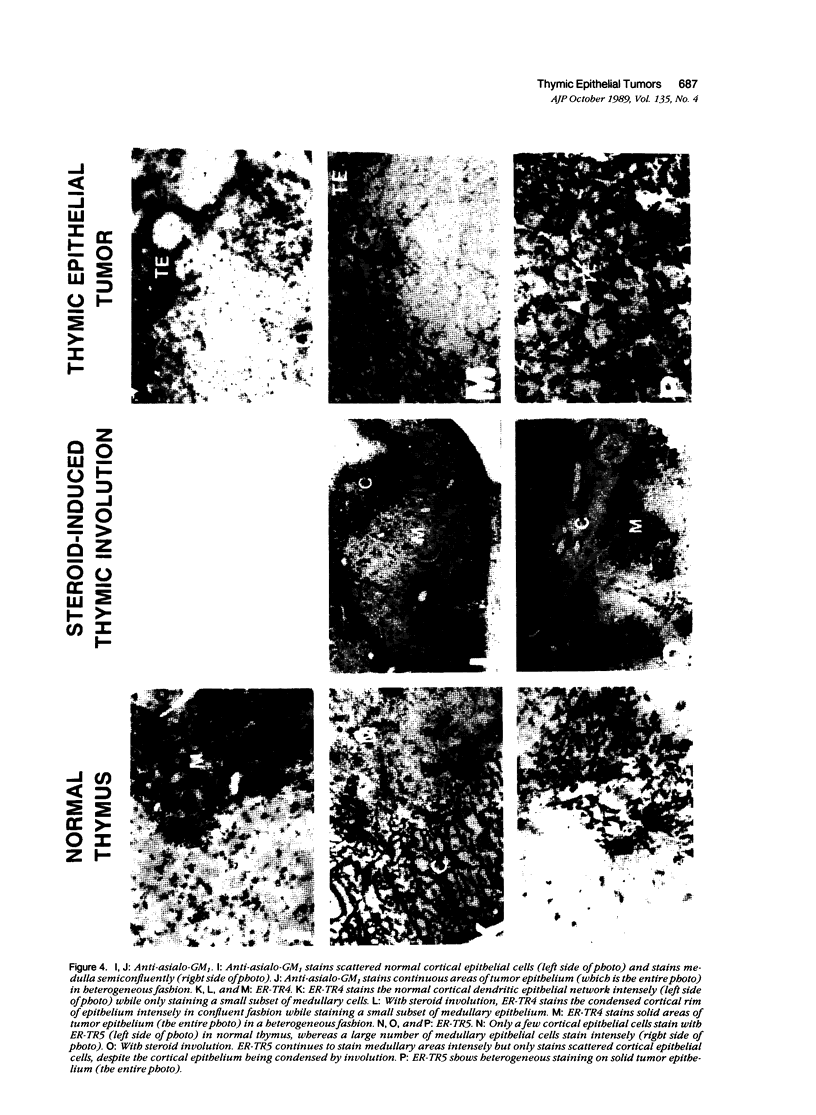
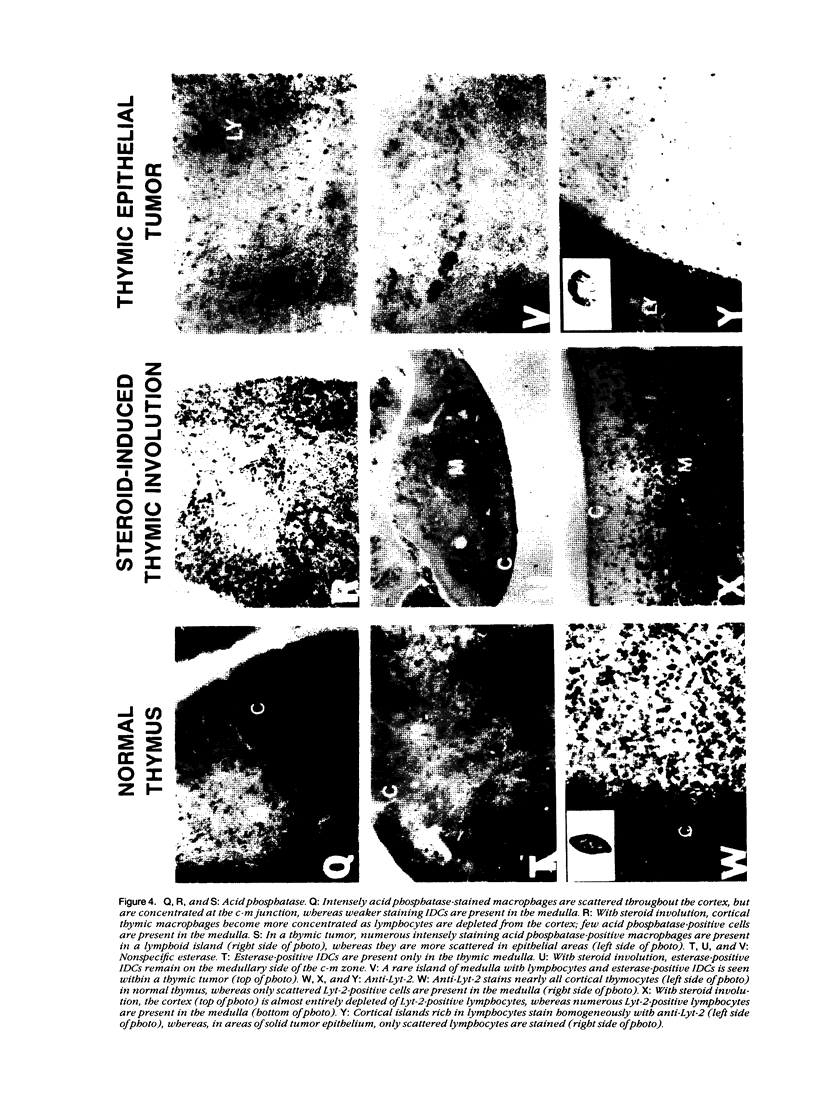
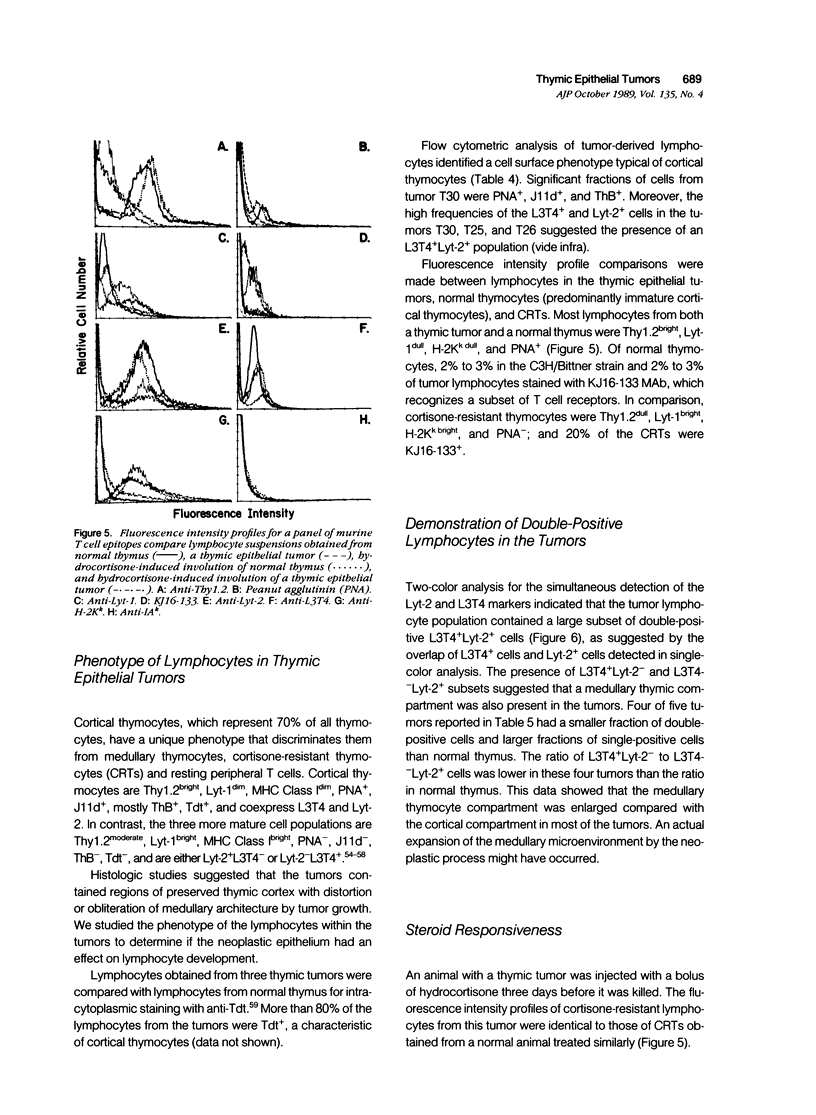
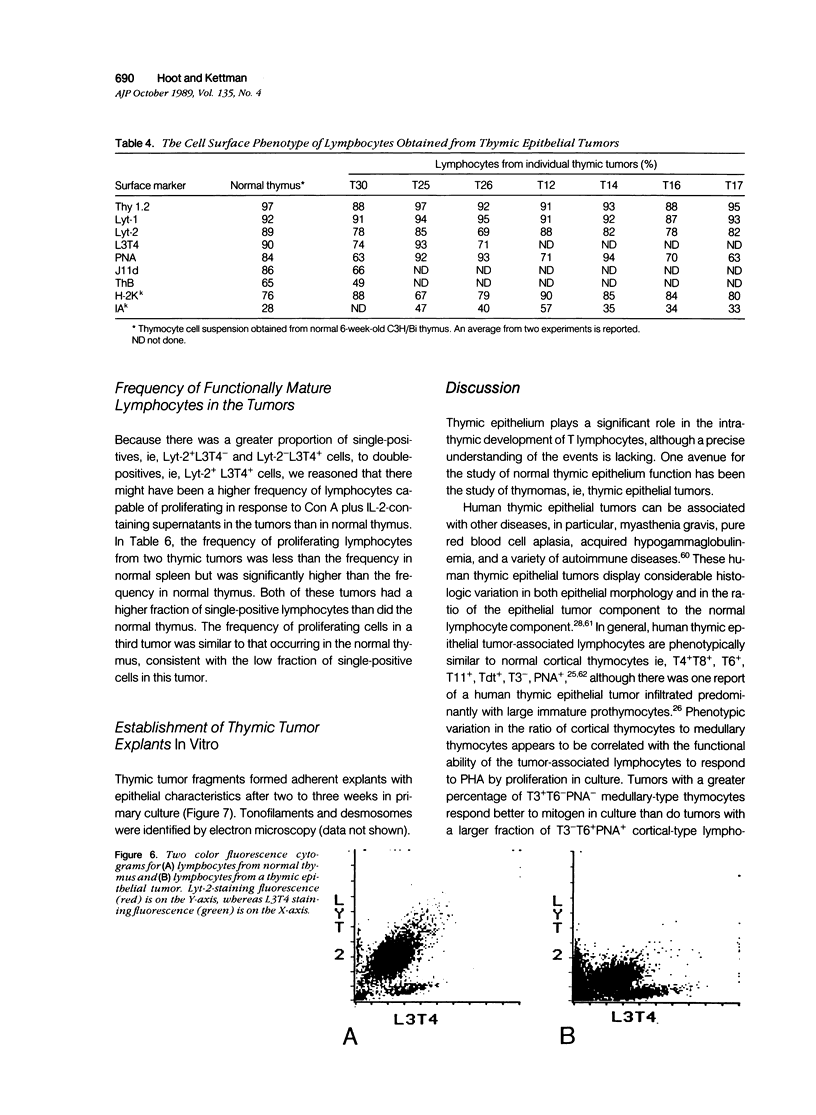
Thymic tumors were induced in C3'/Bittner mice by neonatal inoculation with polyoma virus. The objective of this study was to identify the phenotypes of the cells within the tumors and to attempt to determine the origin of the neoplastic cell population(s). At the ultrastructural level, the neoplastic cells resembled normal thymic epithelium with tonofilaments and desmosomes. Immunoperoxidase staining demonstrated the presence of cytokeratin, Iak, -beta 2-microglobulin, -asialo-GM1, the thymic cortical epithelial marker ER-TR4, and the medullary epithelial marker ER-TR5. Islands of normal cortical thymocytes supported by residual normal cortical epithelium and acid phosphatase-positive cortical macrophages were interspersed in the tumors. Residual islands of normal medullary architecture with nonspecific esterase-positive IDCs were rarely identified in tumors. Most lymphocytes in the tumors were normal immature cortical thymocytes with the phenotype Tdt+, PNA+, Thy 1.2bright, Ly-1dull, H-2Kkdull, ThB+, J11d+, and Lyt-2+L3T4+. Lymphocytes in the tumors were steroid-sensitive like normal thymocytes. The proportions of Lyt-2+L3T4- and Lyt-2-L3T4+ cells were generally larger in the tumors than in normal thymus and reflected the higher frequency of lymphocytes in the tumors capable of proliferating in vitro in response to Con A plus IL-2. The data were consistent with the hypothesis that the neoplasia originates from thymic epithelium that is interspersed with normal, developing thymic lymphocytes.
Full text
PDF


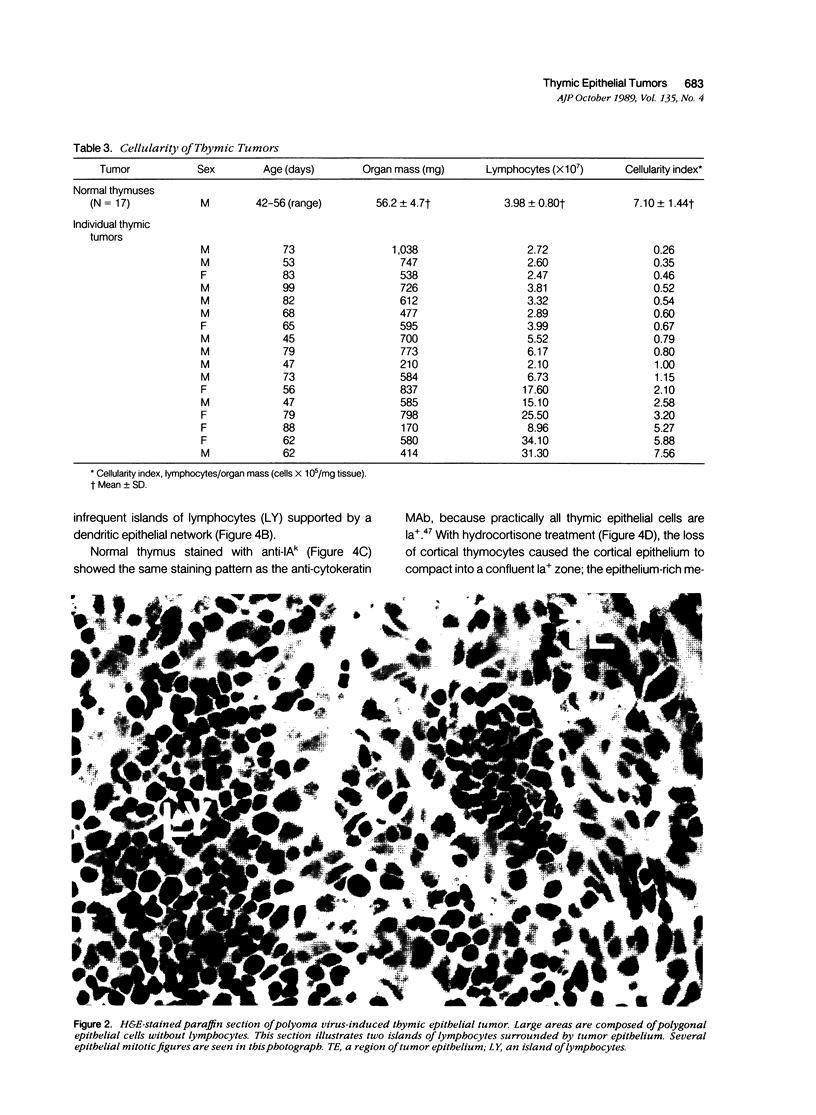





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUFFETT R. F., COMMERFORD S. L., FURTH J., HUNTER M. J. Agent in Ak leukemic tissues, not sedimented at 105, 000 g, causing neoplastic and non-neoplastic lesions. Proc Soc Exp Biol Med. 1958 Nov;99(2):401–407. doi: 10.3181/00379727-99-24364. [DOI] [PubMed] [Google Scholar]
- Battifora H., Sun T. T., Bahu R. M., Rao S. The use of antikeratin antiserum as a diagnostic tool: thymoma versus lymphoma. Hum Pathol. 1980 Nov;11(6):635–641. doi: 10.1016/s0046-8177(80)80074-8. [DOI] [PubMed] [Google Scholar]
- Bockman D. E., Stutman O. Fine structure of a transplanted chemically induced nonlymphoid thymoma. Cancer Res. 1969 Sep;29(9):1663–1668. [PubMed] [Google Scholar]
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- Chilosi M., Iannucci A. M., Pizzolo G., Menestrina F., Fiore-Donati L., Janossy G. Immunohistochemical analysis of thymoma. Evidence for medullary origin of epithelial cells. Am J Surg Pathol. 1984 Apr;8(4):309–318. doi: 10.1097/00000478-198404000-00009. [DOI] [PubMed] [Google Scholar]
- Chollet P., Plagne R., Fonck Y., Chassagne J., Bétail G., Dardenne M., Bach J. F. Thymoma with hypersecretion of thymic hormone. Thymus. 1981 Dec;3(6):321–334. [PubMed] [Google Scholar]
- Chorney M., Shen F. W., Michaelson J., Boyse E. A. Monoclonal antibody to an alloantigenic determinant on beta2-microglobulin (beta 2M) of the mouse. Immunogenetics. 1982;16(1):91–93. doi: 10.1007/BF00364446. [DOI] [PubMed] [Google Scholar]
- DAWE C. J., LAW L. W., DUNN T. B. Studies of parotid-tumor agent in cultures of leukemic tissues of mice. J Natl Cancer Inst. 1959 Oct;23:717–797. [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A. M., Barclay A. N. Identification of the bone marrow-derived ia positive cells in the rat thymus: a morphological and cytochemical study. J Leukoc Biol. 1984 Nov;36(5):561–568. doi: 10.1002/jlb.36.5.561. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A. M., Köhler Y. G., Hoefsmit E. C. Interdigitating cells and macrophages in the acute involuting rat thymus. An electron-microscopic study on phagocytic activity and population development. Cell Tissue Res. 1982;224(2):291–301. doi: 10.1007/BF00216874. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A. M., Sminia T., Köhler Y. G., Janse E. M., Hoefsmit E. C. Ontogeny of the rat thymus micro-environment: development of the interdigitating cell and macrophage populations. Dev Comp Immunol. 1984 Spring;8(2):451–460. doi: 10.1016/0145-305x(84)90052-1. [DOI] [PubMed] [Google Scholar]
- Edwards P. A. Heterogeneous expression of cell-surface antigens in normal epithelia and their tumours, revealed by monoclonal antibodies. Br J Cancer. 1985 Feb;51(2):149–160. doi: 10.1038/bjc.1985.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman J., Trial J., Tonkonogy S., Flaherty L. The Qa2 subregion controls the expression of two antigens recognized by H-2-unrestricted cytotoxic T cells. J Exp Med. 1982 Mar 1;155(3):749–767. doi: 10.1084/jem.155.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gregoire K. E., Barton R. W., Bollum F. J. Demonstration of terminal deoxynucleotidyl transferase in thymocytes by immunofluorescence. Proc Natl Acad Sci U S A. 1977 Feb;74(2):734–738. doi: 10.1073/pnas.74.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Haskins K., Hannum C., White J., Roehm N., Kubo R., Kappler J., Marrack P. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. VI. An antibody to a receptor allotype. J Exp Med. 1984 Aug 1;160(2):452–471. doi: 10.1084/jem.160.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Scearce R. M., Lobach D. F., Hensley L. L. Phenotypic characterization and ontogeny of mesodermal-derived and endocrine epithelial components of the human thymic microenvironment. J Exp Med. 1984 Apr 1;159(4):1149–1168. doi: 10.1084/jem.159.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Warren R. W., Buckley R. H., McClure J. E., Goldstein A. L., Henderson F. W., Hensley L. L., Eisenbarth G. S. Demonstration of abnormalities in expression of thymic epithelial surface antigens in severe cellular immunodeficiency diseases. J Immunol. 1983 Mar;130(3):1182–1188. [PubMed] [Google Scholar]
- Hiai H., Nishi Y., Miyazawa T., Matsudaira Y., Nishizuka Y. Mouse lymphoid leukemias: symbiotic complexes of neoplastic lymphocytes and their microenvironments. J Natl Cancer Inst. 1981 Apr;66(4):713–722. [PubMed] [Google Scholar]
- Horan P. K., Kappler J. W. Automated fluorescent analysis for cytotoxicity assays. J Immunol Methods. 1977;18(3-4):309–316. doi: 10.1016/0022-1759(77)90184-3. [DOI] [PubMed] [Google Scholar]
- Kettman J. R., Soederberg A., Lefkovits I. Mitogenic activation of B cells in vitro: the properties of adherent accessory cells as revealed by partition analysis. J Immunol. 1986 Aug 15;137(4):1144–1148. [PubMed] [Google Scholar]
- Kruisbeek A. M., Bridges S., Carmen J., Longo D. L., Mond J. J. In vivo treatment of neonatal mice with anti-I-A antibodies interferes with the development of the class I, class II, and Mls-reactive proliferating T cell subset. J Immunol. 1985 Jun;134(6):3597–3604. [PubMed] [Google Scholar]
- Kyewski B. A., Rouse R. V., Kaplan H. S. Thymocyte rosettes: multicellular complexes of lymphocytes and bone marrow-derived stromal cells in the mouse thymus. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5646–5650. doi: 10.1073/pnas.79.18.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyewski B. A. Seeding of thymic microenvironments defined by distinct thymocyte-stromal cell interactions is developmentally controlled. J Exp Med. 1987 Aug 1;166(2):520–538. doi: 10.1084/jem.166.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauriola L., Maggiano N., Marino M., Carbone A., Piantelli M., Musiani P. Human thymoma: immunologic characteristics of the lymphocytic component. Cancer. 1981 Nov 1;48(9):1992–1995. doi: 10.1002/1097-0142(19811101)48:9<1992::aid-cncr2820480914>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Lauriola L., Musiani P., Ranelletti F. O., Maggiano N., Piantelli M. Human thymoma lymphocyte mitogenesis: glucocorticoid inhibitory capacity as a function of the size of the more mature T cell subset. Clin Exp Immunol. 1983 Jun;52(3):477–484. [PMC free article] [PubMed] [Google Scholar]
- Law L. W., Dunn T. B., Trainin N., Levey R. H. Studies of thymic function. Wistar Inst Symp Monogr. 1964 Aug;2:105–120. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Manconi P. E., Ennas M. G., Paghi L., Tedde A., Bistrusso A., Masia G., Costa G. Alpha-naphthyl acetate esterase activity in mouse thymus and other lymphoid organs. Thymus. 1982 May;4(3):135–146. [PubMed] [Google Scholar]
- Mathieson B. J., Fowlkes B. J. Cell surface antigen expression on thymocytes: development and phenotypic differentiation of intrathymic subsets. Immunol Rev. 1984 Dec;82:141–173. doi: 10.1111/j.1600-065x.1984.tb01121.x. [DOI] [PubMed] [Google Scholar]
- McFarland E. J., Scearce R. M., Haynes B. F. The human thymic microenvironment: cortical thymic epithelium is an antigenically distinct region of the thymic microenvironment. J Immunol. 1984 Sep;133(3):1241–1249. [PubMed] [Google Scholar]
- Milićević N. M., Milićević Z. J. Enzyme-histochemical characterization of macrophages in the rat thymus, with special reference to metallophilic cells of the corticomedullary zone. J Leukoc Biol. 1984 Dec;36(6):761–769. doi: 10.1002/jlb.36.6.761. [DOI] [PubMed] [Google Scholar]
- Mokhtar N., Hsu S. M., Lad R. P., Haynes B. F., Jaffe E. S. Thymoma: lymphoid and epithelial components mirror the phenotype of normal thymus. Hum Pathol. 1984 Apr;15(4):378–384. doi: 10.1016/s0046-8177(84)80037-4. [DOI] [PubMed] [Google Scholar]
- Musiani P., Lauriola L., Maggiano N., Tonali P., Piantelli M. Functional properties of human thymoma lymphocytes: role of subcellular factors in blastic activation. J Natl Cancer Inst. 1982 Oct;69(4):827–831. [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Piantelli M., Ranelletti F. O., Musiani P., Lauriola L., Maggiano N. A human thymoma with prothymocyte-like infiltration. Clin Immunol Immunopathol. 1983 Sep;28(3):350–360. doi: 10.1016/0090-1229(83)90102-2. [DOI] [PubMed] [Google Scholar]
- Rosai J. "Lymphoepithelioma-like" thymic carcinoma: another tumor related to Epstein-Barr virus? N Engl J Med. 1985 May 16;312(20):1320–1322. doi: 10.1056/NEJM198505163122010. [DOI] [PubMed] [Google Scholar]
- SMITH C. Studies on thymus of the mammal. IX. Histochemical study of irradiated mouse thymus. Proc Soc Exp Biol Med. 1956 Nov;93(2):310–314. doi: 10.3181/00379727-93-22740. [DOI] [PubMed] [Google Scholar]
- STEWART S. E., EDDY B. E., BORGESE N. Neoplasms in mice inoculated with a tumor agent carried in tissue culture. J Natl Cancer Inst. 1958 Jun;20(6):1223–1243. doi: 10.1093/jnci/20.6.1223. [DOI] [PubMed] [Google Scholar]
- STEWART S. E., EDDY B. E., GOCHENOUR A. M., BORGESE N. G., GRUBBS G. E. The induction of neoplasms with a substance released from mouse tumors by tissue culture. Virology. 1957 Apr;3(2):380–400. doi: 10.1016/0042-6822(57)90100-9. [DOI] [PubMed] [Google Scholar]
- Salyer W. R., Eggleston J. C. Thymoma: a clinical and pathological study of 65 cases. Cancer. 1976 Jan;37(1):229–249. doi: 10.1002/1097-0142(197601)37:1<229::aid-cncr2820370133>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Savino W., Berrih S., Dardenne M. Thymic epithelial antigen, acquired during ontogeny and defined by the anti-p19 monoclonal antibody, is lost in thymomas. Lab Invest. 1984 Sep;51(3):292–296. [PubMed] [Google Scholar]
- Scollay R., Shortman K. Identification of early stages of T lymphocyte development in the thymus cortex and medulla. J Immunol. 1985 Jun;134(6):3632–3642. [PubMed] [Google Scholar]
- Scollay R., Shortman K. Thymocyte subpopulations: an experimental review, including flow cytometric cross-correlations between the major murine thymocyte markers. Thymus. 1983 Sep;5(5-6):245–295. [PubMed] [Google Scholar]
- Singer A., Hathcock K. S., Hodes R. J. Self recognition in allogeneic thymic chimeras. Self recognition by T helper cells from thymus-engrafted nude mice is restricted to the thymic H-2 haplotype. J Exp Med. 1982 Jan 1;155(1):339–344. doi: 10.1084/jem.155.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutman O., Yunis E. J., Good R. A. Carcinogen-induced tumors of the thymus. 3. Restoration of neonatally thymectomized mice with thymomas in cell-impermeable chambers. J Natl Cancer Inst. 1969 Aug;43(2):499–508. [PubMed] [Google Scholar]
- Stutman O., Yunis E. J., Good R. A. Carcinogen-induced tumors of the thymus. II. Lung colonies as a means of separating different cell types of a functional thymoma. J Natl Cancer Inst. 1969 May;42(5):783–795. [PubMed] [Google Scholar]
- Stutman O., Yunis E. J., Good R. A. Carcinogen-induced tumors of the thymus. IV. Humoral influences of normal thymus and functional thymomas and influence of posthymectomy period on restoration. J Exp Med. 1969 Oct 1;130(4):809–819. doi: 10.1084/jem.130.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tridente G. Immunopathology of the human thymus. Semin Hematol. 1985 Jan;22(1):56–67. [PubMed] [Google Scholar]
- Van Ewijk W. Immunohistology of lymphoid and non-lymphoid cells in the thymus in relation to T lymphocyte differentiation. Am J Anat. 1984 Jul;170(3):311–330. doi: 10.1002/aja.1001700307. [DOI] [PubMed] [Google Scholar]
- Van Ewijk W., Rouse R. V., Weissman I. L. Distribution of H-2 microenvironments in the mouse thymus. Immunoelectron microscopic identification of I-A and H-2K bearing cells. J Histochem Cytochem. 1980 Oct;28(10):1089–1099. doi: 10.1177/28.10.6999083. [DOI] [PubMed] [Google Scholar]
- Van Vliet E., Melis M., Van Ewijk W. Monoclonal antibodies to stromal cell types of the mouse thymus. Eur J Immunol. 1984 Jun;14(6):524–529. doi: 10.1002/eji.1830140608. [DOI] [PubMed] [Google Scholar]
- Wekerle H., Ketelsen U. P. Thymic nurse cells--Ia-bearing epithelium involved in T-lymphocyte differentiation? Nature. 1980 Jan 24;283(5745):402–404. doi: 10.1038/283402a0. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Callahan G. N., Althage A., Cooper S., Klein P. A., Klein J. On the thymus in the differentiation of "H-2 self-recognition" by T cells: evidence for dual recognition? J Exp Med. 1978 Mar 1;147(3):882–896. doi: 10.1084/jem.147.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maagd R. A., MacKenzie W. A., Schuurman H. J., Ritter M. A., Price K. M., Broekhuizen R., Kater L. The human thymus microenvironment: heterogeneity detected by monoclonal anti-epithelial cell antibodies. Immunology. 1985 Apr;54(4):745–754. [PMC free article] [PubMed] [Google Scholar]
- van Ewijk W., van Soest P. L., van den Engh G. J. Fluorescence analysis and anatomic distribution of mouse T lymphocyte subsets defined by monoclonal antibodies to the antigens Thy-1, Lyt-1, Lyt-2, and T-200. J Immunol. 1981 Dec;127(6):2594–2604. [PubMed] [Google Scholar]