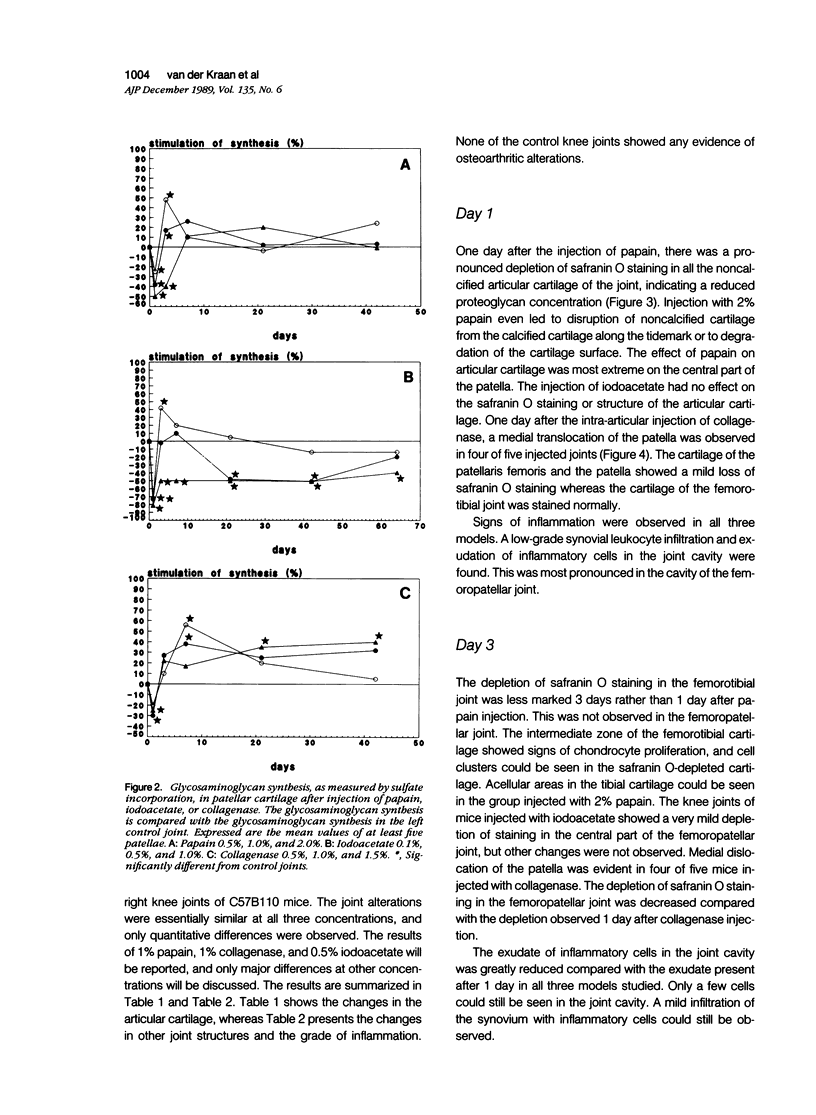
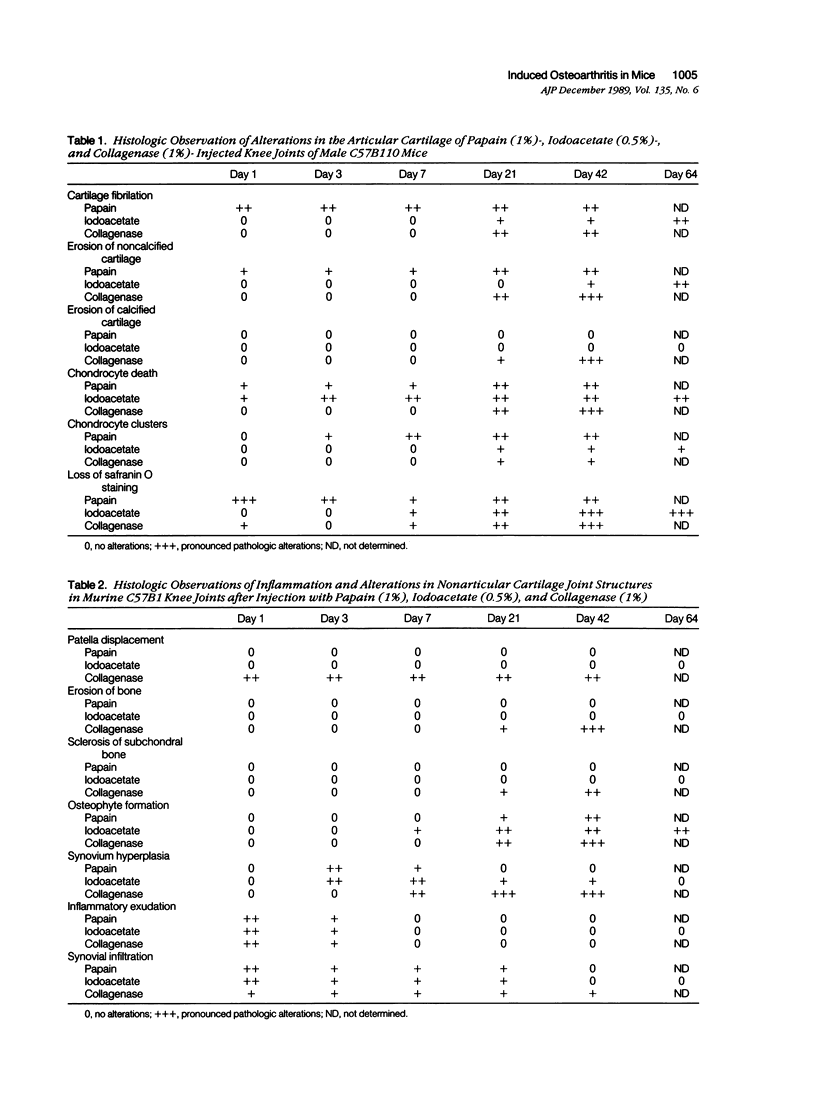
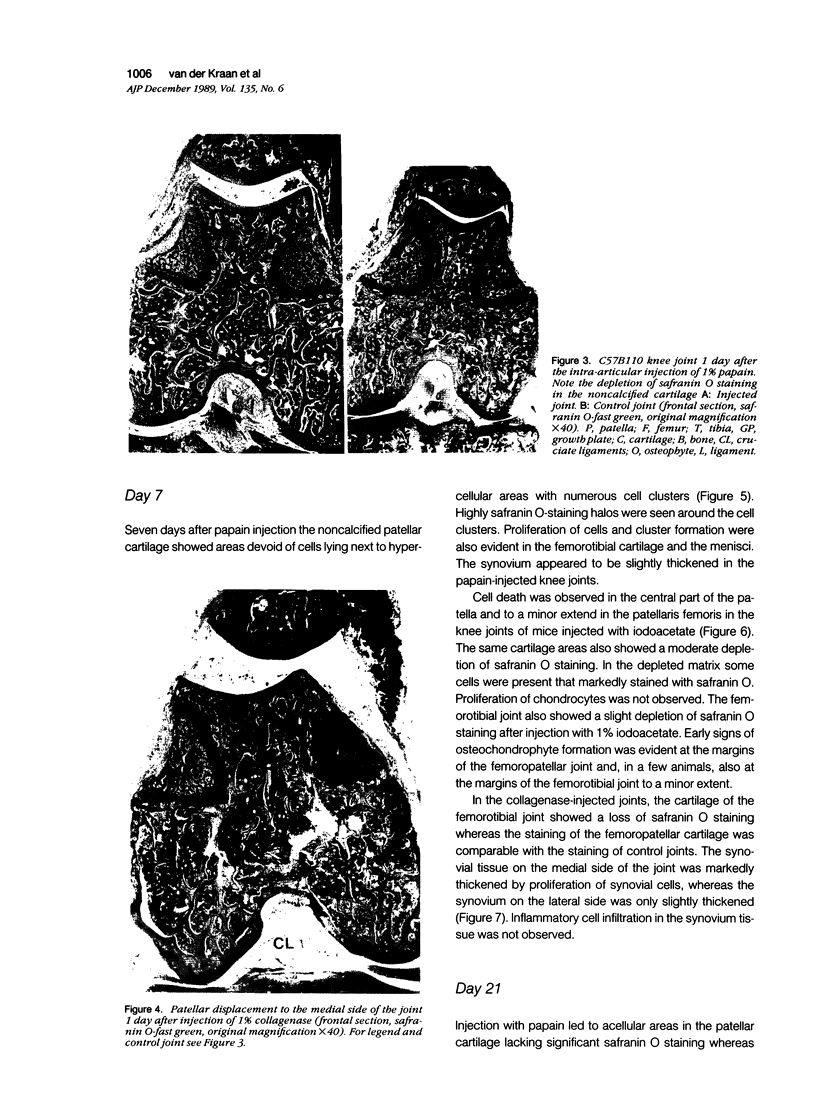
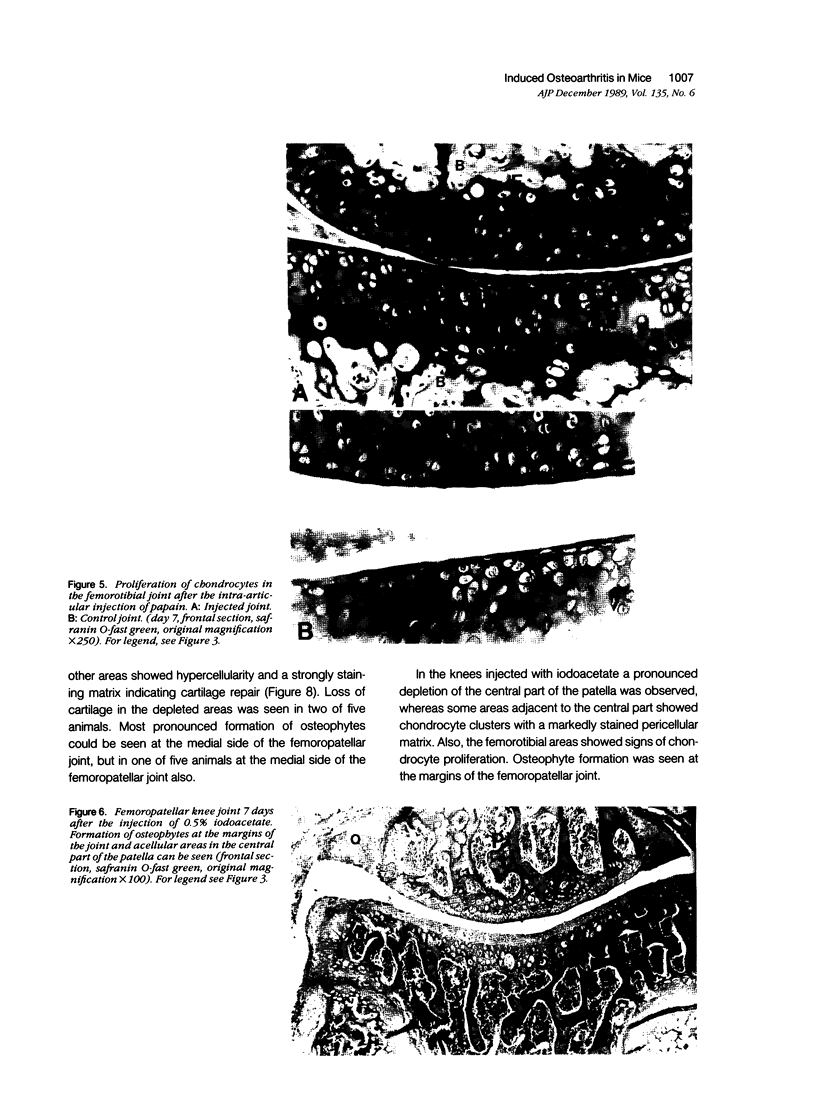
Abstract
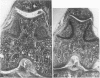
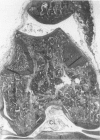
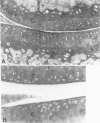
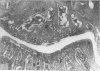
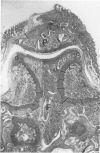
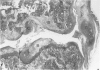
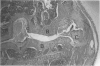
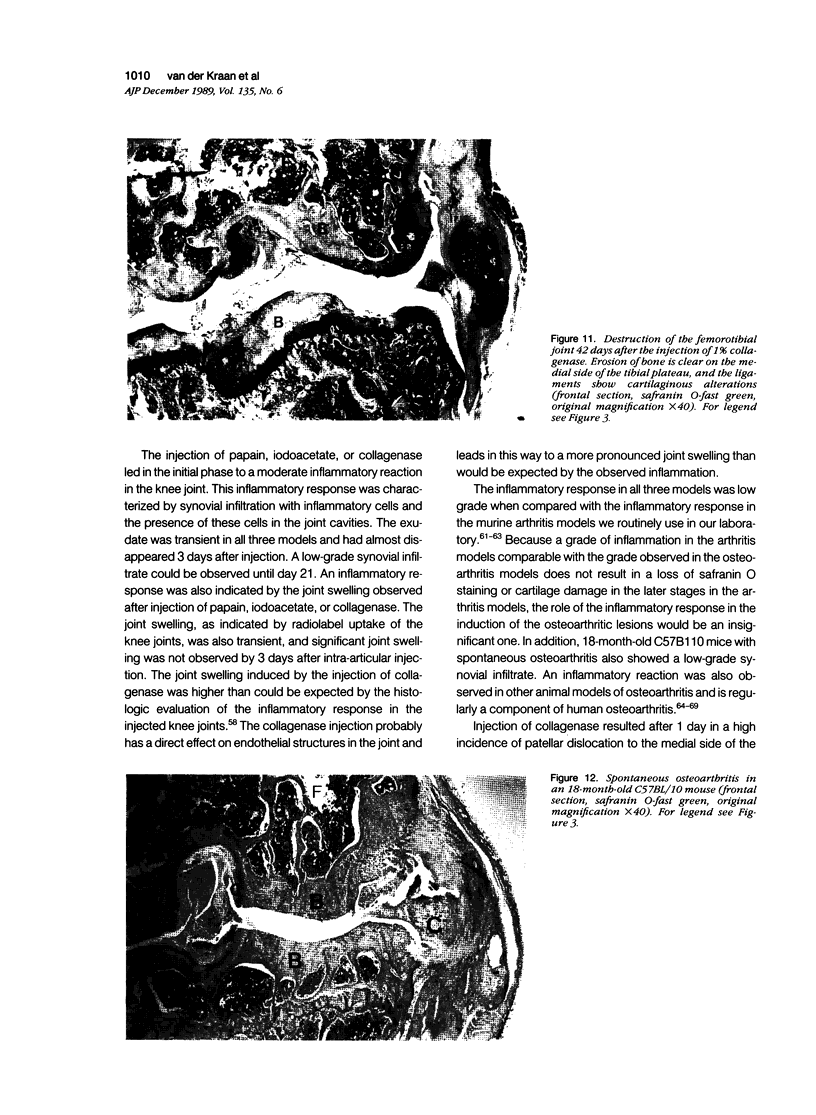
Male, 10-week-old C57B1 10 mice received a single intraarticular injection in the knee joints with papain, iodoacetate, or collagenase. This led to osteoarthritic lesions, such as matrix depletion, chondrocyte proliferation, and osteophyte formation, in the injected knee joints within several weeks. After injection of iodoacetate and papain, the main osteoarthritic alterations were localized in the femoropatellar joint, whereas injection of collagenase led to marked osteoarthritic lesions in the femorotibial joint. The mechanism of induction of these alterations appears to differ for iodoacetate and papain on one site and collagenase on the other site. Data are presented that collagenase injection, by way of damaging ligaments and tendons, destabilizes the knee joint eventually leading to osteoarthritic alterations. In contrast, injection of papain or iodoacetate directly interferes with cartilage metabolism resulting in osteoarthritic changes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman F. P. A metabolic dysfunction in early murine osteoarthritis. Ann Rheum Dis. 1981 Jun;40(3):303–306. doi: 10.1136/ard.40.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman R. D., Fries J. F., Bloch D. A., Carstens J., Cooke T. D., Genant H., Gofton P., Groth H., McShane D. J., Murphy W. A. Radiographic assessment of progression in osteoarthritis. Arthritis Rheum. 1987 Nov;30(11):1214–1225. doi: 10.1002/art.1780301103. [DOI] [PubMed] [Google Scholar]
- Arsever C. L., Bole G. G. Experimental osteoarthritis induced by selective myectomy and tendotomy. Arthritis Rheum. 1986 Feb;29(2):251–261. doi: 10.1002/art.1780290214. [DOI] [PubMed] [Google Scholar]
- Bendele A. M., Hulman J. F. Spontaneous cartilage degeneration in guinea pigs. Arthritis Rheum. 1988 Apr;31(4):561–565. doi: 10.1002/art.1780310416. [DOI] [PubMed] [Google Scholar]
- Bendele A. M. Progressive chronic osteoarthritis in femorotibial joints of partial medial meniscectomized guinea pigs. Vet Pathol. 1987 Sep;24(5):444–448. doi: 10.1177/030098588702400512. [DOI] [PubMed] [Google Scholar]
- Bentley G. Papain-induced degenerative arthritis of the hip in rabbits. J Bone Joint Surg Br. 1971 May;53(2):324–337. [PubMed] [Google Scholar]
- Bingel S. A., Sande R. D., Wight T. N. Undersulfated chondroitin sulfate in cartilage from a miniature poodle with spondyloepiphyseal dysplasia. Connect Tissue Res. 1986;15(4):283–302. doi: 10.3109/03008208609001986. [DOI] [PubMed] [Google Scholar]
- Brocklehurst R., Bayliss M. T., Maroudas A., Coysh H. L., Freeman M. A., Revell P. A., Ali S. Y. The composition of normal and osteoarthritic articular cartilage from human knee joints. With special reference to unicompartmental replacement and osteotomy of the knee. J Bone Joint Surg Am. 1984 Jan;66(1):95–106. [PubMed] [Google Scholar]
- Cantatore F. P., Benazzo F., Ribatti D., Lapadula G., D'Amico S., Tursi A., Pipitone V. Early alteration of synovial membrane in osteoarthrosis. Clin Rheumatol. 1988 Jun;7(2):214–219. doi: 10.1007/BF02204457. [DOI] [PubMed] [Google Scholar]
- Carney S. L., Billingham M. E., Muir H., Sandy J. D. Structure of newly synthesised (35S)-proteoglycans and (35S)-proteoglycan turnover products of cartilage explant cultures from dogs with experimental osteoarthritis. J Orthop Res. 1985;3(2):140–147. doi: 10.1002/jor.1100030203. [DOI] [PubMed] [Google Scholar]
- Colombo C., Butler M., O'Byrne E., Hickman L., Swartzendruber D., Selwyn M., Steinetz B. A new model of osteoarthritis in rabbits. I. Development of knee joint pathology following lateral meniscectomy and section of the fibular collateral and sesamoid ligaments. Arthritis Rheum. 1983 Jul;26(7):875–886. doi: 10.1002/art.1780260709. [DOI] [PubMed] [Google Scholar]
- Cooke T. D. Immune pathology in polyarticular osteoarthritis. Clin Orthop Relat Res. 1986 Dec;(213):41–49. [PubMed] [Google Scholar]
- Coulais Y., Marcelon G., Cros J., Guiraud R. Etude d'un modèle expérimental d'arthrose. I.--Induction et étude ultrastructurale. Pathol Biol (Paris) 1983 Sep;31(7):577–582. [PubMed] [Google Scholar]
- Cox M. J., McDevitt C. A., Arnoczky S. P., Warren R. F. Changes in the chondroitin sulfate-rich region of articular cartilage proteoglycans in experimental osteoarthritis. Biochim Biophys Acta. 1985 Jun 18;840(2):228–234. doi: 10.1016/0304-4165(85)90123-0. [DOI] [PubMed] [Google Scholar]
- Farkas T., Bihari-Varga M., Biró T. Thermoanalytical and histological study of intra-articular papain-induced degradation and repair of rabbit cartilage. I. Immature animals. Ann Rheum Dis. 1974 Jul;33(4):385–390. doi: 10.1136/ard.33.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas T., Bihari-Varga M., Biró T. Thermoanalytical and histological study of intra-articular papain-induced degradation and repair of rabbit cartilage. II. Mature animals. Ann Rheum Dis. 1976 Feb;35(1):23–26. doi: 10.1136/ard.35.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg D. L., Egan M. S., Cohen A. S. Inflammatory synovitis in degenerative joint disease. J Rheumatol. 1982 Mar-Apr;9(2):204–209. [PubMed] [Google Scholar]
- Goldenberg D. L., Egan M. S., Cohen A. S. Inflammatory synovitis in degenerative joint disease. J Rheumatol. 1982 Mar-Apr;9(2):204–209. [PubMed] [Google Scholar]
- Havdrup T., Telhag H. Papain-induced changes in the knee joints of adult rabbits. Acta Orthop Scand. 1977;48(2):143–149. doi: 10.3109/17453677708985125. [DOI] [PubMed] [Google Scholar]
- Inerot S., Heinegård D., Audell L., Olsson S. E. Articular-cartilage proteoglycans in aging and osteoarthritis. Biochem J. 1978 Jan 1;169(1):143–156. doi: 10.1042/bj1690143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAY G. E., Jr, SOKOLOFF L. Natural history of degenerative joint disease in small laboratory animals. II. Epiphyseal maturation and osteoarthritis of the knee of mice of inbred strains. AMA Arch Pathol. 1956 Aug;62(2):129–135. [PubMed] [Google Scholar]
- Kalbhen D. A., Scherbach E., Felten K. Histological investigations of the antiarthrotic effect of tribenoside (Glyvenol) in experimental osteoarthrosis. J Rheumatol. 1983 Apr;10(2):267–281. [PubMed] [Google Scholar]
- Katona G. Osteoarthritis--an inflammatory disease? Int J Tissue React. 1984;6(6):453–461. [PubMed] [Google Scholar]
- Kruijsen M. W., van den Berg W. B., van de Putte L. B. Influence of the severity and duration of murine antigen-induced arthritis on cartilage proteoglycan synthesis and chondrocyte death. Arthritis Rheum. 1985 Jul;28(7):813–819. doi: 10.1002/art.1780280713. [DOI] [PubMed] [Google Scholar]
- Kruijsen M. W., van den Berg W. B., van de Putte L. B. Sequential alterations of periarticular structures in antigen-induced arthritis in mice. Histological observations on fibrous capsule, ligaments, bone and muscles, using whole joint sections. Br J Exp Pathol. 1983 Jun;64(3):298–305. [PMC free article] [PubMed] [Google Scholar]
- Kruijsen M. W., van den Berg W. B., van de Putte L. B., van den Broek W. J. Detection and quantification of experimental joint inflammation in mice by measurement of 99mTc-pertechnetate uptake. Agents Actions. 1981 Dec;11(6-7):640–642. doi: 10.1007/BF01978775. [DOI] [PubMed] [Google Scholar]
- Lindblad S., Hedfors E. Arthroscopic and immunohistologic characterization of knee joint synovitis in osteoarthritis. Arthritis Rheum. 1987 Oct;30(10):1081–1088. doi: 10.1002/art.1780301001. [DOI] [PubMed] [Google Scholar]
- Lindblad S., Hedfors E. Arthroscopic and immunohistologic characterization of knee joint synovitis in osteoarthritis. Arthritis Rheum. 1987 Oct;30(10):1081–1088. doi: 10.1002/art.1780301001. [DOI] [PubMed] [Google Scholar]
- Lukoschek M., Schaffler M. B., Burr D. B., Boyd R. D., Radin E. L. Synovial membrane and cartilage changes in experimental osteoarthrosis. J Orthop Res. 1988;6(4):475–492. doi: 10.1002/jor.1100060403. [DOI] [PubMed] [Google Scholar]
- MURRAY D. G. EXPERIMENTALLY INDUCED ARTHRITIS USING INTRA-ARTICULAR PAPAIN. Arthritis Rheum. 1964 Jun;7:211–219. doi: 10.1002/art.1780070304. [DOI] [PubMed] [Google Scholar]
- Manicourt D. H., Pita J. C. Progressive depletion of hyaluronic acid in early experimental osteoarthritis in dogs. Arthritis Rheum. 1988 Apr;31(4):538–544. doi: 10.1002/art.1780310411. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Mankin H. J., Johnson M. E., Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. III. Distribution and metabolism of amino sugar-containing macromolecules. J Bone Joint Surg Am. 1981 Jan;63(1):131–139. [PubMed] [Google Scholar]
- Mankin H. J., Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Joint Surg Am. 1970 Apr;52(3):424–434. [PubMed] [Google Scholar]
- McDevitt C., Gilbertson E., Muir H. An experimental model of osteoarthritis; early morphological and biochemical changes. J Bone Joint Surg Br. 1977 Feb;59(1):24–35. doi: 10.1302/0301-620X.59B1.576611. [DOI] [PubMed] [Google Scholar]
- Moriizumi T., Yamashita N., Okada Y. Papain-induced changes in the guinea pig knee joint with special reference to cartilage healing. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(6):461–474. doi: 10.1007/BF02899052. [DOI] [PubMed] [Google Scholar]
- Moskowitz R. W., Davis W., Sammarco J., Martens M., Baker J., Mayor M., Burstein A. H., Frankel V. H. Experimentally induced degenerative joint lesions following partial meniscectomy in the rabbit. Arthritis Rheum. 1973 May-Jun;16(3):397–405. doi: 10.1002/art.1780160317. [DOI] [PubMed] [Google Scholar]
- Moskowitz R. W., Goldberg V. M. Osteophyte evolution: studies in an experimental partial meniscectomy model. J Rheumatol. 1987 May;14(Spec No):116–118. [PubMed] [Google Scholar]
- Moskowitz R. W., Howell D. S., Goldberg V. M., Muniz O., Pita J. C. Cartilage proteoglycan alterations in an experimentally induced model of rabbit osteoarthritis. Arthritis Rheum. 1979 Feb;22(2):155–163. doi: 10.1002/art.1780220208. [DOI] [PubMed] [Google Scholar]
- Orford C. R., Gardner D. L., O'Connor P., Bates G., Swallow J. J., Brito-Babapulle L. A. Ultrastructural alterations in glycosaminoglycans of dog femoral condylar cartilage after surgical division of an anterior cruciate ligament: a study with cupromeronic blue in a critical electrolyte concentration technique. J Anat. 1986 Oct;148:233–244. [PMC free article] [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J., Altman R. D., Ghandur-Mnaymneh L., Howell D. S., Woessner J. F., Jr Collagenolytic activity and collagen matrix breakdown of the articular cartilage in the Pond-Nuki dog model of osteoarthritis. Arthritis Rheum. 1983 Jul;26(7):866–874. doi: 10.1002/art.1780260708. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J. Cartilage degradation by neutral proteoglycanases in experimental osteoarthritis. Suppression by steroids. Arthritis Rheum. 1985 Dec;28(12):1393–1401. doi: 10.1002/art.1780281212. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J., Ghandur-Mnaymneh L., Howell D. S., Woessner J. F., Jr Role of synovial membrane inflammation in cartilage matrix breakdown in the Pond-Nuki dog model of osteoarthritis. Arthritis Rheum. 1985 May;28(5):554–561. doi: 10.1002/art.1780280515. [DOI] [PubMed] [Google Scholar]
- Peyron J. G. Osteoarthritis. The epidemiologic viewpoint. Clin Orthop Relat Res. 1986 Dec;(213):13–19. [PubMed] [Google Scholar]
- Pidd J. G., Gardner D. L., Adams M. E. Ultrastructural changes in the femoral condylar cartilage of mature American foxhounds following transection of the anterior cruciate ligament. J Rheumatol. 1988 Apr;15(4):663–669. [PubMed] [Google Scholar]
- Revell P. A., Mayston V., Lalor P., Mapp P. The synovial membrane in osteoarthritis: a histological study including the characterisation of the cellular infiltrate present in inflammatory osteoarthritis using monoclonal antibodies. Ann Rheum Dis. 1988 Apr;47(4):300–307. doi: 10.1136/ard.47.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostand K. S., Baker J. R., Caterson B., Christner J. E. Articular cartilage proteoglycans from normal and osteoarthritic mice. Arthritis Rheum. 1986 Jan;29(1):95–105. doi: 10.1002/art.1780290113. [DOI] [PubMed] [Google Scholar]
- SOKOLOFF L. Natural history of degenerative joint disease in small laboratory animals. I. Pathological anatomy of degenerative joint disease in mice. AMA Arch Pathol. 1956 Aug;62(2):118–128. [PubMed] [Google Scholar]
- Sandy J. D., Adams M. E., Billingham M. E., Plaas A., Muir H. In vivo and in vitro stimulation of chondrocyte biosynthetic activity in early experimental osteoarthritis. Arthritis Rheum. 1984 Apr;27(4):388–397. doi: 10.1002/art.1780270405. [DOI] [PubMed] [Google Scholar]
- Santer V., White R. J., Roughley P. J. Proteoglycans from normal and degenerate cartilage of the adult human tibial plateau. Arthritis Rheum. 1981 May;24(5):691–700. doi: 10.1002/art.1780240510. [DOI] [PubMed] [Google Scholar]
- Schalkwijk J., van den Berg W. B., van de Putte L. B., Joosten L. A. Hydrogen peroxide suppresses the proteoglycan synthesis of intact articular cartilage. J Rheumatol. 1985 Apr;12(2):205–210. [PubMed] [Google Scholar]
- Scheck M., Sakovich L. Degenerative joint disease of the canine hip: experimental production by multiple papain and prednisone injections. Clin Orthop Relat Res. 1972 Jul-Aug;86:115–120. doi: 10.1097/00003086-197207000-00017. [DOI] [PubMed] [Google Scholar]
- Schwartz E. R., Leveille C. R., Stevens J. W., Oh W. H. Proteoglycan structure and metabolism in normal and osteoarthritic cartilage of guinea pigs. Arthritis Rheum. 1981 Dec;24(12):1528–1539. doi: 10.1002/art.1780241212. [DOI] [PubMed] [Google Scholar]
- Schwartz E. R., Oh W. H., Leveille C. R. Experimentally induced osteoarthritis in guinea pigs: metabolic responses in articular cartilage to developing pathology. Arthritis Rheum. 1981 Nov;24(11):1345–1355. doi: 10.1002/art.1780241103. [DOI] [PubMed] [Google Scholar]
- Schünke M., Tillmann B., Brück M., Müller-Ruchholtz W. Morphologic characteristics of developing osteoarthrotic lesions in the knee cartilage of STR/IN mice. Arthritis Rheum. 1988 Jul;31(7):898–905. doi: 10.1002/art.1780310711. [DOI] [PubMed] [Google Scholar]
- Shaw N. E., Lacey E. The influence of corticosteroids on normal and papain-treated articular cartilage in the rabbit. J Bone Joint Surg Br. 1973 Feb;55(1):197–205. [PubMed] [Google Scholar]
- Telhag H., Lindberg L. A method for inducing osteoarthritic changes in rabbits' knees. Clin Orthop Relat Res. 1972 Jul-Aug;86:214–223. doi: 10.1097/00003086-197207000-00033. [DOI] [PubMed] [Google Scholar]
- Teshima R., Treadwell B. V., Trahan C. A., Mankin H. J. Comparative rates of proteoglycan synthesis and size of proteoglycans in normal and osteoarthritic chondrocytes. Arthritis Rheum. 1983 Oct;26(10):1225–1230. doi: 10.1002/art.1780261009. [DOI] [PubMed] [Google Scholar]
- Vignon E., Arlot M., Hartmann D., Moyen B., Ville G. Hypertrophic repair of articular cartilage in experimental osteoarthrosis. Ann Rheum Dis. 1983 Feb;42(1):82–88. doi: 10.1136/ard.42.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton M. Degenerative joint disease in the mouse knee; histological observations. J Pathol. 1977 Oct;123(2):109–122. doi: 10.1002/path.1711230207. [DOI] [PubMed] [Google Scholar]
- Walton M. Degenerative joint disease in the mouse knee; radiological and morphological observations. J Pathol. 1977 Oct;123(2):97–107. doi: 10.1002/path.1711230206. [DOI] [PubMed] [Google Scholar]
- Walton M. Patella displacement and osteoarthrosis of the knee joint in mice. J Pathol. 1979 Apr;127(4):165–172. doi: 10.1002/path.1711270402. [DOI] [PubMed] [Google Scholar]
- Walton M. Studies of degenerative joint disease in the mouse knee joint; scanning electron microscopy. J Pathol. 1977 Dec;123(4):211–217. doi: 10.1002/path.1711230403. [DOI] [PubMed] [Google Scholar]
- Wilhelmi G., Faust R. Suitability of the C57 black mouse as an experimental animal for the study of skeletal changes due to ageing, with special reference to osteo-arthrosis and its response to tribenoside. Pharmacology. 1976;14(4):289–296. doi: 10.1159/000136607. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Brandt K. D. Exercise increases osteophyte formation and diminishes fibrillation following chemically induced articular cartilage injury. J Anat. 1984 Dec;139(Pt 4):599–611. [PMC free article] [PubMed] [Google Scholar]
- Williams J. M., Brandt K. D. Immobilization ameliorates chemically-induced articular cartilage damage. Arthritis Rheum. 1984 Feb;27(2):208–216. doi: 10.1002/art.1780270213. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Brandt K. D. Temporary immobilisation facilitates repair of chemically induced articular cartilage injury. J Anat. 1984 May;138(Pt 3):435–446. [PMC free article] [PubMed] [Google Scholar]
- de Vries B. J., van den Berg W. B., Vitters E., van de Putte L. B. Quantitation of glycosaminoglycan metabolism in anatomically intact articular cartilage of the mouse patella: in vitro and in vivo studies with 35S-sulfate, 3H-glucosamine, and 3H-acetate. Rheumatol Int. 1986;6(6):273–281. doi: 10.1007/BF00541319. [DOI] [PubMed] [Google Scholar]
- van den Berg W. B., Kruijsen M. W., van de Putte L. B., van Beusekom H. J., van der Sluis-van der Pol M., Zwarts W. A. Antigen-induced and zymosan-induced arthritis in mice: studies on in vivo cartilage proteoglycan synthesis and chondrocyte death. Br J Exp Pathol. 1981 Jun;62(3):308–316. [PMC free article] [PubMed] [Google Scholar]