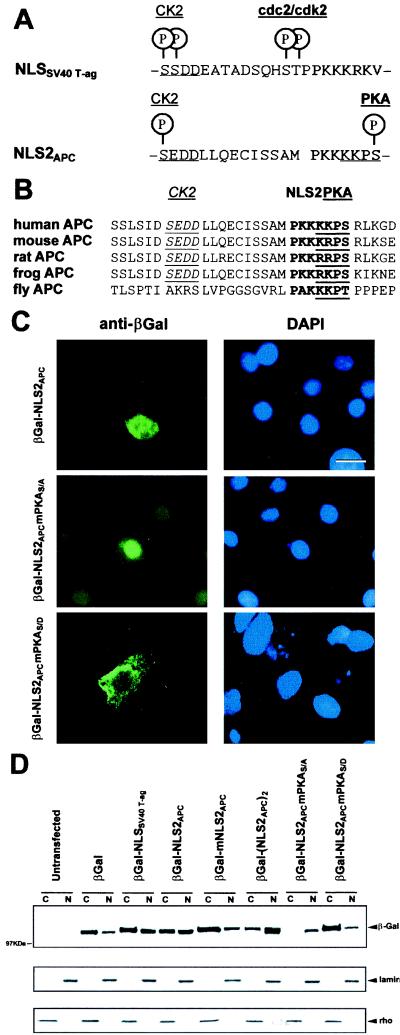
Figure 4.
NLS2APC-mediated nuclear translocation of βGal chimera is regulated by phosphorylation at a PKA site. (A) Alignment of NLS2APC with NLSSV40 T-ag reveals similarity of NLS sequence as well as the adjacent putative phosphorylation sites. (B) The NLS2APC region is highly conserved among different species. NLS2 sequences are bold; the CK2 and PKA sites are underlined. (C) βGal-NLS2APC localizes to both the cytoplasm and nucleus of L cells. βGal-NLS2APCmPKAS/A is predominantly nuclear and βGal-NLS2APCmPKAS/D predominantly cytoplasmic. (Bar = 10 μm.) Cells were scored as above, and the incidence of nuclear staining for each βGal fusion is as follows: βGal-NLS2APC, 69(±8)%; βGal-NLS2APCmPKAS/A, 100(±0)%; and βGal-NLS2APCmPKAS/D, 29(±6)%. (D) Fractionation and Western immunoblot analysis of 293 cells transiently transfected with various constructs confirmed the subcellular localization of the chimeric proteins. Fractions are labeled as follows: C, cytosol; N, nuclear. The various cell fractions were characterized by striping and reprobing the blot for the marker proteins: lamin as a nuclear marker and rho as a cytoplasmic marker.