Abstract
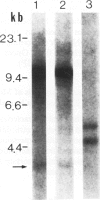
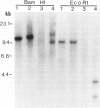
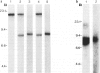
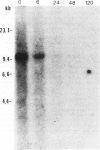
Studies on immunoglobulin gene rearrangement in lymphoid lesions are an increasingly important application of molecular biology in diagnostic medicine. The authors have therefore examined the possibility of detecting such rearrangements in formalin-fixed, paraffin-embedded pathology specimens. Southern blots of DNA obtained from optimally fixed tissues were very similar to blots of unfixed material, except that the electrophoretic mobility of the fixed DNA fragments was sometimes slightly reduced. High-molecular-weight DNA was not recovered from suboptimally fixed partially autolysed samples. Increasing the time of exposure to formalin resulted in loss of hybridizable DNA. Monoclonal rearrangements of heavy and light chain immunoglobulin genes could be detected in formalin-fixed specimens provided that these fixation artifacts were taken into consideration. This technique expands the pool of material available for studies on gene rearrangement and should facilitate the use of such studies in clinical medicine.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C., Krontiris T. G., Mak T. W., Wilkes B. M. Rearrangement of the gene for the beta chain of the T-cell receptor in T-cell chronic lymphocytic leukemia and related disorders. N Engl J Med. 1985 Aug 29;313(9):529–533. doi: 10.1056/NEJM198508293130901. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Enea V., Bothwell A. L., Baltimore D. Activity of multiple light chain genes in murine myeloma cells producing a single, functional light chain. Cell. 1980 Aug;21(1):1–12. doi: 10.1016/0092-8674(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Arnold A., Cossman J., Bakhshi A., Jaffe E. S., Waldmann T. A., Korsmeyer S. J. Immunoglobulin-gene rearrangements as unique clonal markers in human lymphoid neoplasms. N Engl J Med. 1983 Dec 29;309(26):1593–1599. doi: 10.1056/NEJM198312293092601. [DOI] [PubMed] [Google Scholar]
- Bertness V., Kirsch I., Hollis G., Johnson B., Bunn P. A., Jr T-cell receptor gene rearrangements as clinical markers of human T-cell lymphomas. N Engl J Med. 1985 Aug 29;313(9):534–538. doi: 10.1056/NEJM198508293130902. [DOI] [PubMed] [Google Scholar]
- Brown N. A., Liu C., Berenson J. R., Garcia C. R., Wang R., Calame K. L. Immunoglobulin JH, C mu, and C gamma gene rearrangements in human B lymphocytes clonally transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1985 Jan;82(2):556–560. doi: 10.1073/pnas.82.2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleclough C., Perry R. P., Karjalainen K., Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981 Apr 2;290(5805):372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- Dubeau L., Chandler L. A., Gralow J. R., Nichols P. W., Jones P. A. Southern blot analysis of DNA extracted from formalin-fixed pathology specimens. Cancer Res. 1986 Jun;46(6):2964–2969. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fishleder A., Tubbs R., Hesse B., Levine H. Uniform detection of immunoglobulin-gene rearrangement in benign lymphoepithelial lesions. N Engl J Med. 1987 Apr 30;316(18):1118–1121. doi: 10.1056/NEJM198704303161803. [DOI] [PubMed] [Google Scholar]
- Goelz S. E., Hamilton S. R., Vogelstein B. Purification of DNA from formaldehyde fixed and paraffin embedded human tissue. Biochem Biophys Res Commun. 1985 Jul 16;130(1):118–126. doi: 10.1016/0006-291x(85)90390-0. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Hollis G. F., Korsmeyer S. J., Waldmann T. A., Leder P. Clustered arrangement of immunoglobulin lambda constant region genes in man. Nature. 1981 Dec 10;294(5841):536–540. doi: 10.1038/294536a0. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Korsmeyer S. J., Waldmann T. A., Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981 Apr 2;290(5805):368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- Jackson V. Studies on histone organization in the nucleosome using formaldehyde as a reversible cross-linking agent. Cell. 1978 Nov;15(3):945–954. doi: 10.1016/0092-8674(78)90278-7. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Sharrow S. O., Goldman C. K., Leder P., Waldmann T. A. Normal human B cells display ordered light chain gene rearrangements and deletions. J Exp Med. 1982 Oct 1;156(4):975–985. doi: 10.1084/jem.156.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N., Nelson J., Meyer P., Lukes R. J., Parker J. W. Reactive lymphoid hyperplasia with single class (monoclonal) surface immunoglobulin. Am J Clin Pathol. 1983 Sep;80(3):300–308. doi: 10.1093/ajcp/80.3.300. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley J. D. Biological implications of consistent chromosome rearrangements in leukemia and lymphoma. Cancer Res. 1984 Aug;44(8):3159–3168. [PubMed] [Google Scholar]
- Samoszuk M. K., Krailo M., Yan Q. H., Lukes R. J., Parker J. W. Limitations of numerical ratios for defining monoclonality of immunoglobulin light chains in B-cell lymphomas. Diagn Immunol. 1985;3(3):133–138. [PubMed] [Google Scholar]
- Taub R. A., Hollis G. F., Hieter P. A., Korsmeyer S., Waldmann T. A., Leder P. Variable amplification of immunoglobulin lambda light-chain genes in human populations. Nature. 1983 Jul 14;304(5922):172–174. doi: 10.1038/304172a0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Davis M. M., Bongiovanni K. F., Korsmeyer S. J. Rearrangements of genes for the antigen receptor on T cells as markers of lineage and clonality in human lymphoid neoplasms. N Engl J Med. 1985 Sep 26;313(13):776–783. doi: 10.1056/NEJM198509263131303. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Hu E., Wood G. S., Moulds C., Cleary M. L., Warnke R., Sklar J. Clonal rearrangements of T-cell receptor genes in mycosis fungoides and dermatopathic lymphadenopathy. N Engl J Med. 1985 Aug 29;313(9):539–544. doi: 10.1056/NEJM198508293130903. [DOI] [PubMed] [Google Scholar]
- Yunis J. J. The chromosomal basis of human neoplasia. Science. 1983 Jul 15;221(4607):227–236. doi: 10.1126/science.6336310. [DOI] [PubMed] [Google Scholar]