Figure 4. S6K1 phosphorylates GSK3 in TSC-deficient cells.
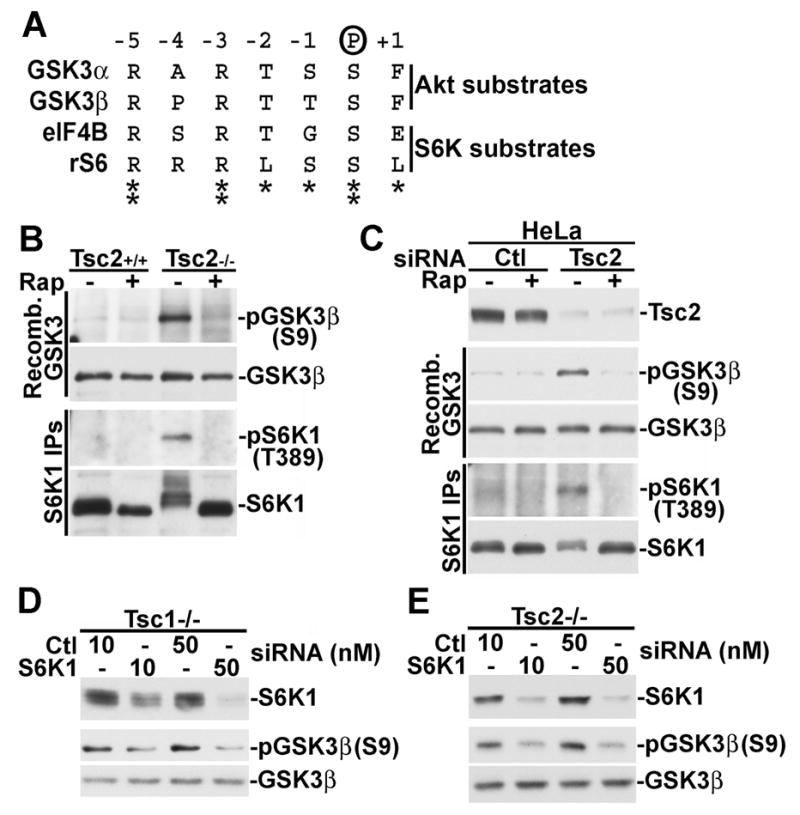
(A) The corresponding phosphorylation sites on GSK3 and the S6K1 substrates eIF4B and S6 are similar. The residues flanking GSK3α-S21, GSK3β-S9, eIF4B-S422, and ribosomal S6-S236 are aligned.
(B) S6K1 from serum-starved Tsc2−/− MEFs, but not wild-type MEFs, can phosphorylate GSK3β-S9 in vitro. Tsc2+/+ and Tsc2−/− MEFs were serum starved for 16 h and then treated for 15 min with 20 nM rapamycin, where indicated. Immunoprecipitated S6K1 was used in an in vitro kinase assay with bacterially produced GSK3β as the substrate. GSK3 phosphorylation on S9 was detected using a phospho-specifc antibody.
(C) S6K1 from serum-starved HeLa cells treated with TSC2 siRNAs, but not control siRNAs, can phosphorylate GSK3β-S9 in vitro. 24 h post-transfection with control or TSC2-targetting siRNAs, HeLa cells were treated as described in B.
(D) S6K1 knockdown in Tsc1−/− cells blocks the constitutive phosphorylation of GSK3. 24 hours post-transfection with the indicated doses of control or S6K1-targetting siRNAs, Tsc1−/− MEFs were serum starved for 16 hours.
(E) Tsc2−/− MEFs were treated as described in D.
