Figure 7. mTORC1-dependent phosphorylation of GSK3 under conditions of cellular insulin resistance.
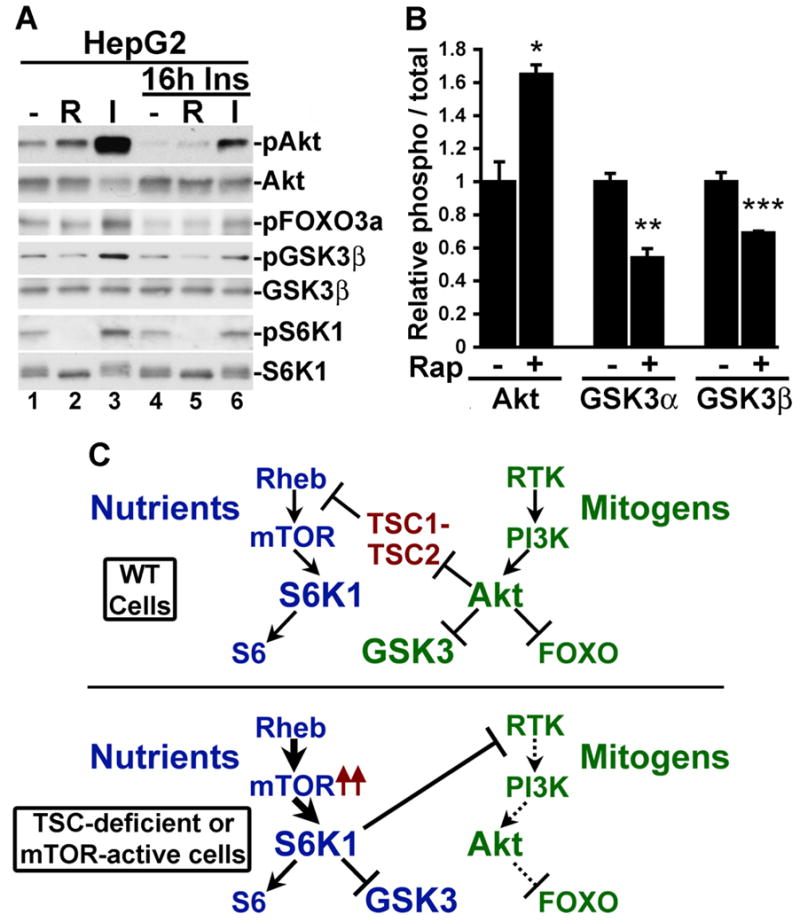
(A) GSK3 phosphorylation is sensitive to rapamycin in insulin-resistant HepG2 cells. HepG2 cells were serum starved for 16 h in the absence or presence of 100 nM insulin and were treated for 15 min with 20 nM rapamycin (R) or 100 nM insulin (I), as indicated.
(B) Rapamycin increases Akt phosphorylation but decreases GSK3 phosphorylation in insulin-resistant HepG2 cells. Cells were treated for 16 h with 100 nM insulin and then treated for 15 min with 20 nM rapamycin. Three independent lysates from each condition were then immunoblotted for phospho-Akt-S473, total Akt, phospho GSK3α/β-S21/S9, and total GSK3α/β, and the data were quantified using an infared imaging system. The data are expressed as the mean +/− SEM relative ratio of phospho-protein to total protein between untreated and rapamycin-treated samples. *p=0.0105; **p=0.0001; ***p=0.0439
(C) Differential regulation of GSK3 by the mitogen-stimulated PI3K-Akt pathway (green) and the nutrient-sensitive mTOR-S6K1 pathway (blue).
