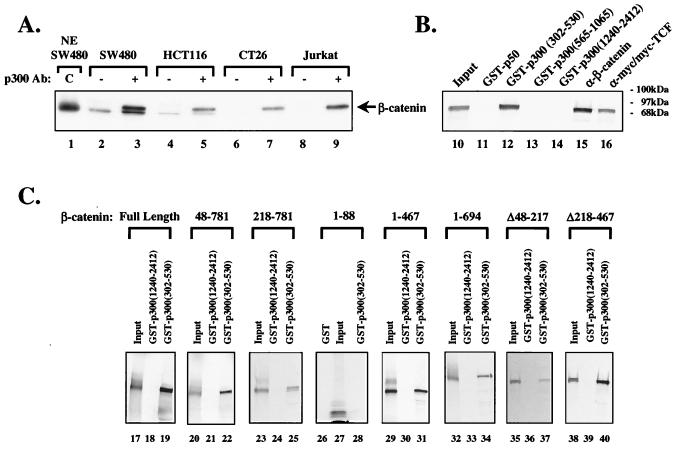
Figure 1.
p300 interacts with β-catenin. (A) β-Catenin coimmunoprecipitates with p300. Nuclear extracts from the indicated cell lines were immunoprecipitated without (−) or with (+) anti-p300 antibody, and immune complexes were resolved by SDS/PAGE, followed by Western blotting with anti-β-catenin antibodies. The arrow indicates the position of β-catenin. NE SW480 represents nuclear extract of SW480 human colon carcinoma cells. Ten percent of the extract used for immunoprecipitation was loaded in the control lane. (B) β-Catenin interacts with the CH1 domain of p300. Different fragments of p300 were fused to GST and used to pull down in vitro translated 35S-labeled β-catenin. GST-p300 (amino acids 302–530), GST-p300 (amino acids 565-1065), and GST-p300 (amino acids 1240–2412) contain residues 302–530, 565-1065, and 1240–2412 of p300, respectively. GST-p300 (amino acids 302–530) contains the CH1 domain of p300. Anti-β-catenin and anti-myc antibodies complexed with myc-tagged TCF were used to bind β-catenin to evaluate the binding strength of GST-p300 (amino acids 302–530). Ten percent of the in vitro translated β-catenin extract used in the pull-down assays was loaded as input. (C) p300 interacts with the NH2- or COOH-terminal regions of β-catenin. The indicated deletion mutants of β-catenin were generated by in vitro transcription and translation and incubated with GST-p300 (amino acids 302–530) as in B. A COOH-terminal fragment of GST-p300 (amino acids 1240–2412) was used as a negative control. Ten percent of the in vitro translated β-catenin extract used in the pull-down assays was loaded as input.