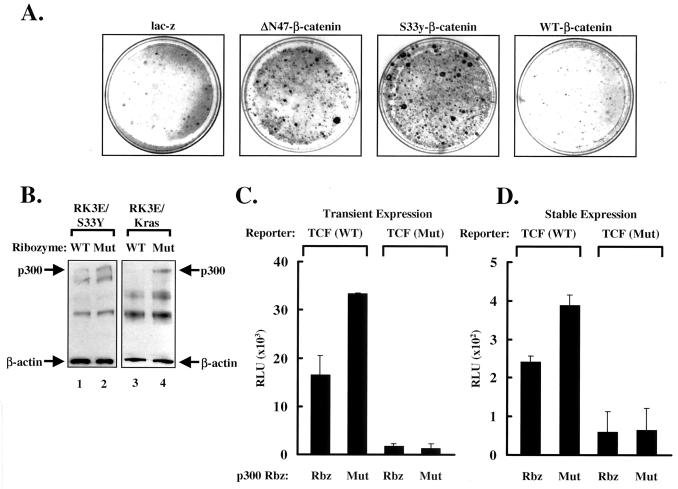
Figure 3.
Reduction of the endogenous p300 by the p300 ribozyme and its effect on β-catenin/TCF-mediated transcription. (A) Focus-formation assays. Immortalized neonatal rat kidney cells RK3E were transduced with retroviruses expressing the lacZ gene (as negative control), ΔN47 (47 residues from the NH2 terminus of β-catenin were deleted), S33Y (point mutation at residue 33 on β-catenin), and wild-type β-catenin. (B) Wild-type p300 ribozyme reduces the endogenous levels of p300. Immortalized neonatal rat kidney cells transformed with a mutant β-catenin (RK3E/S33Y) or K-ras (RK3E/Kras) were transduced with retroviruses expressing wild-type or mutant p300 ribozyme. After 7 days of G418 selection, the cells were harvested and resolved by SDS/PAGE followed by Western blotting. Anti-p300 antibody was used to detect the endogenous p300 protein and anti-β-actin antibody was used to detect endogenous β-actin, which serves as an internal control. The arrows indicate the position of p300 or β-actin. (C) Reduction of transcription of the TCF reporter by transient expression of the p300 ribozyme (Rbz). RK3E/S33Y cells were transfected with wild-type or mutant TCF reporter and a plasmid expressing wild-type or mutant p300 ribozyme. The RSV-β-gal construct was included in all transfections as a control for transfection efficiency. The cells were harvested 24 h after transfection and assayed for the luciferase activity (relative light units, RLU). Luciferase activity was normalized against β-gal activity. (D) Reduction of transcription of the TCF reporter by stably expressing p300 ribozyme. The RK3E/S33Y cells were transduced with retroviruses expressing wild-type or mutant p300 ribozyme. After 7 days of G418 selection, the ribozyme-transduced cells were transfected with the wild-type or mutant TCF reporter. The cells were harvested and the luciferase activity (RLU) was measured and calculated as in B.