Abstract
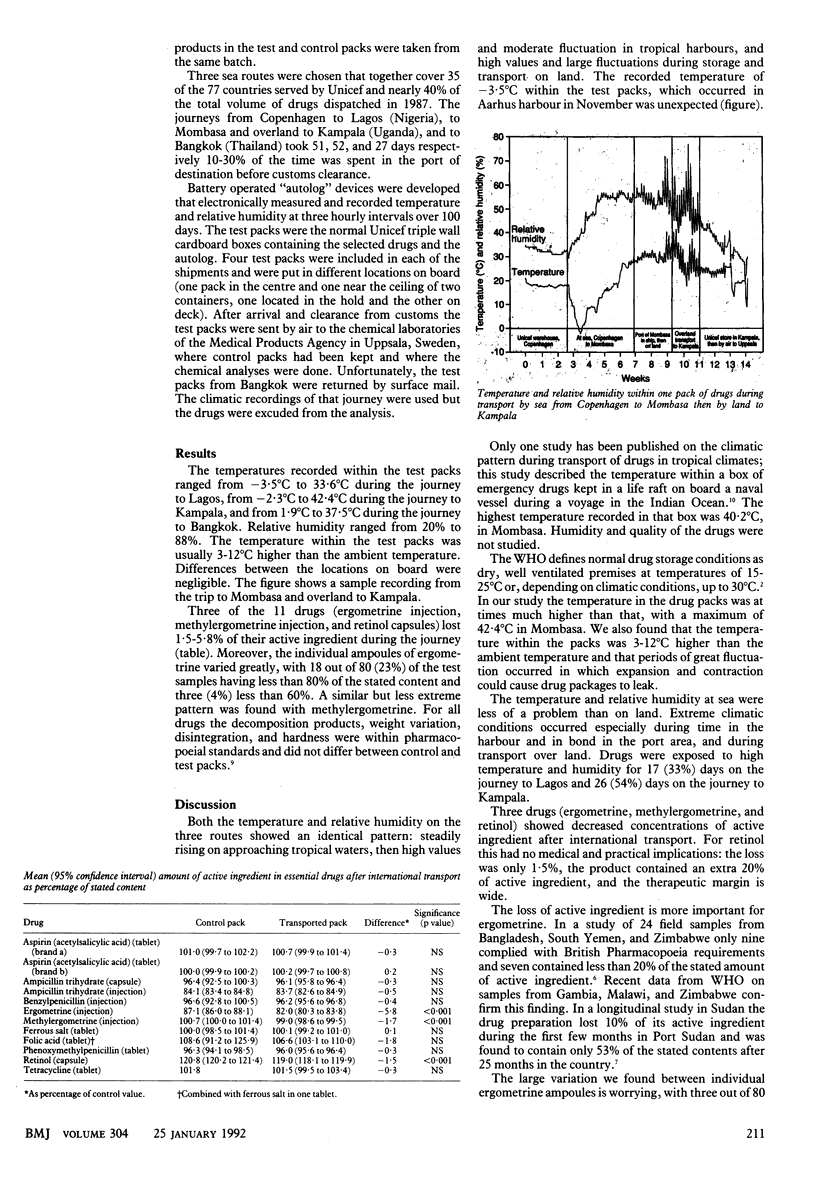
OBJECTIVE--To determine whether present methods of international transport of essential drugs by sea adversely affect their quality. DESIGN--Controlled longitudinal study of drug shipments sent by sea from Unicef in Copenhagen to Lagos; to Mombasa and by land to Kampala; and to Bangkok. 11 essential drugs were stored in four locations on board the ships. SETTING--Main shipping routes from Unicef, Copenhagen, to tropical countries. MAIN OUTCOME MEASURES--Temperature and relative humidity in the test packs during the journey. Amount of active ingredient in the drugs before and after shipment. RESULTS--Temperatures recorded within the test packs range from -3.5 degrees C to 42.4 degrees C and were 3-12 degrees C higher than the ambient temperature. Relative humidity within the packs ranged from 20% to 88%. Differences between the locations on board were negligible. Ergometrine injection, methylergometrine injection, and retinol capsules lost 1.5-5.8% of their activity. Ampoules of ergometrine showed a large variation in the amount of active ingredient after shipment, with three of 80 samples having concentrations 60% below those stated. Ampicillin, benzylpenicillin, phenoxymethylpenicillin, and tetracycline were not affected by transport. CONCLUSIONS--Drugs were exposed to a much higher temperature and humidity than is recommended by the manufacturer, especially in tropical harbours and during inland transport. Except for ergometrine and methylergometrine the transport would not affect clinical effectiveness.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hogerzeil H. V., Battersby A., Srdanovic V., Stjernstrom N. E. Stability of essential drugs during shipment to the tropics. BMJ. 1992 Jan 25;304(6821):210–212. doi: 10.1136/bmj.304.6821.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogerzeil H. V., de Goeje M. J., Abu-Reid I. O. Stability of essential drugs in Sudan. Lancet. 1991 Sep 21;338(8769):754–755. doi: 10.1016/0140-6736(91)91470-f. [DOI] [PubMed] [Google Scholar]
- Pawełczyk E., Hermann T., Zajac M., Knitter B., Smiłowski B. Kinetics of drug decomposition. Part 61. Kinetics of various ampicillin forms degradation in solid phase. Pol J Pharmacol Pharm. 1980 Jan-Feb;32(1):47–54. [PubMed] [Google Scholar]
- Pawełczyk E., Płotkowiak Z., Knitter K., Kozakiewicz-Wegner B. Kinetics of drug decomposition. Part 62. Kinetics of penicillin G potassium salt (PGP) thermal degradation in solid phase. Pol J Pharmacol Pharm. 1980 Jan-Feb;32(1):55–62. [PubMed] [Google Scholar]
- Pikal M. J., Lukes A. L., Lang J. E. Thermal decomposition of amorphous beta-lactam antibacterials. J Pharm Sci. 1977 Sep;66(9):1312–1316. doi: 10.1002/jps.2600660927. [DOI] [PubMed] [Google Scholar]
- Walker G. J., Hogerzeil H. V. Potency of ergometrine in tropical countries. Lancet. 1988 Aug 13;2(8607):393–393. doi: 10.1016/s0140-6736(88)92858-9. [DOI] [PubMed] [Google Scholar]
- York P. The shelf life of some antibiotic preparations stored under tropical conditions. Pharmazie. 1977 Feb;32(2):101–104. [PubMed] [Google Scholar]


