Abstract
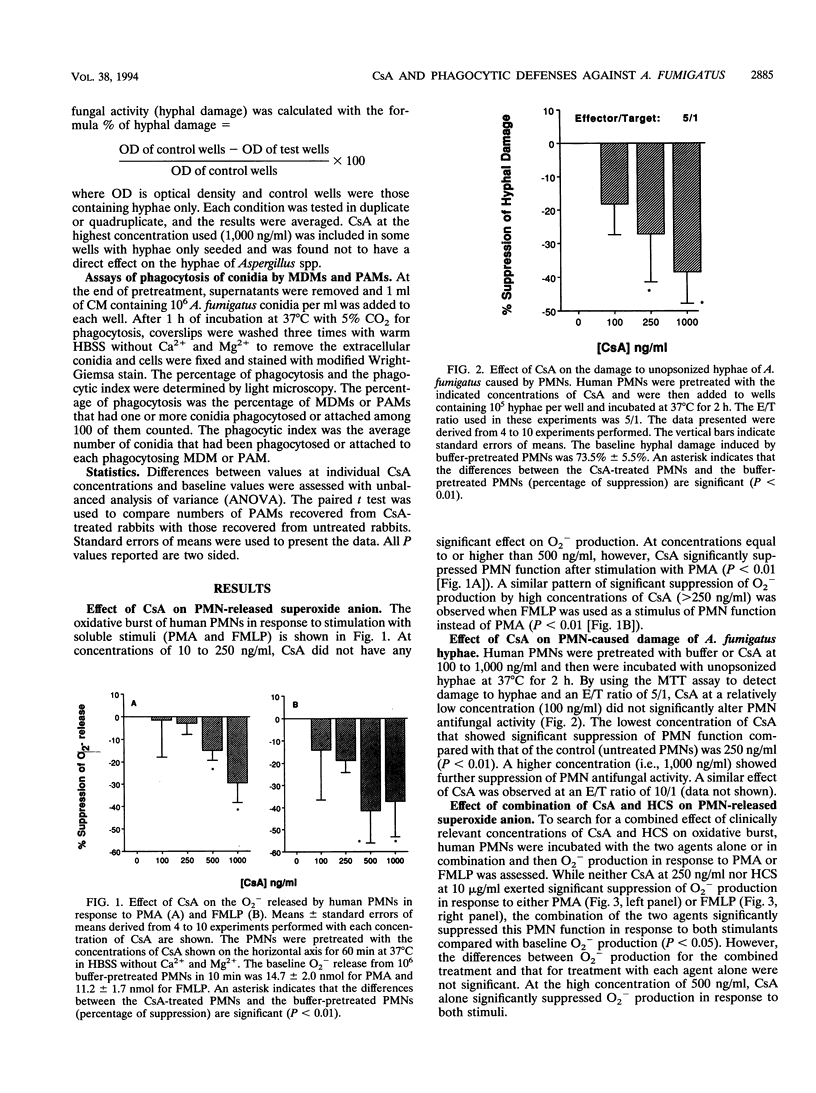
Because cyclosporin A (CsA) is extensively used as an immunosuppressive agent, its effects on phagocytic defenses against Aspergillus fumigatus were studied in vitro and ex vivo. After incubation with 10 to 250 ng of CsA per ml at 37 degrees C for 60 min, polymorphonuclear leukocytes (PMNs) exhibited unaltered superoxide anion (O2-) production in response to phorbol myristate acetate and N-formylmethionyl leucyl phenylalanine, whereas > or = 500 ng/ml significantly suppressed it (P < 0.01). Moreover, at < 250 ng of CsA per ml, PMNs exhibited no change in their capacity to damage unopsonized hyphae of A. fumigatus compared with controls, whereas at > or = 250 ng/ml, CsA suppressed the function (P < 0.01). Although neither CsA (250 ng/ml) nor hydrocortisone (10 micrograms/ml) suppressed PMN O2- production in response to phorbol myristate acetate and N-formylmethionyl leucyl phenylalanine, combination of the two agents reduced the function compared with that at the baseline (P < 0.05). Incubation of monocytes with 100 ng of CsA per ml for 1 or 2 days suppressed their antihyphal activity. No essential change in phagocytic activity of monocyte-derived macrophages (MDMs) against A. fumigatus conidia, tested as the percentage of phagocytosing MDMs and average number of MDM-associated conidia, was detected after 2 or 4 days of incubation with 10 to 1,000 ng of CsA per ml. Furthermore, in rabbits treated with CsA (up to 20 mg/kg of body weight per day intravenously for 7 days), neither O2- production and hyphal damage caused by PMNs or monocytes against hyphae nor phagocytosis of conidia by pulmonary alveolar macrophages was significantly suppressed. Thus, these results demonstrated that CsA within therapeutically relevant concentrations does not suppress antifungal activity of phagocytes except that of circulating monocytes. However, it may induce significant immunosuppression of phagocytes' antifungal function at relatively high concentrations in vitro, especially when combined with corticosteroids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsen T. G., Carter C. S., Read E. J., Rubin M., Goetzman H. G., Lizzio E. F., Lee Y. L., Hanson M., Pizzo P. A., Hoffman T. Stimulatory effect of counterflow centrifugal elutriation in large-scale separation of peripheral blood monocytes can be reversed by storing the cells at 37 degrees C. J Clin Apher. 1991;6(1):48–53. doi: 10.1002/jca.2920060110. [DOI] [PubMed] [Google Scholar]
- Brooks R. G., Hofflin J. M., Jamieson S. W., Stinson E. B., Remington J. S. Infectious complications in heart-lung transplant recipients. Am J Med. 1985 Oct;79(4):412–422. doi: 10.1016/0002-9343(85)90027-0. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Epstein B., Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. A possible host defense mechanism in aspergillosis and mucormycosis. Am J Pathol. 1978 May;91(2):313–328. [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978 Feb;61(2):349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drath D. B., Kahan B. D. Alterations in rat pulmonary macrophage function by the immunosuppressive agents cyclosporine, azathioprine, and prednisolone. Transplantation. 1983 Jun;35(6):588–592. doi: 10.1097/00007890-198306000-00014. [DOI] [PubMed] [Google Scholar]
- Dummer J. S., Hardy A., Poorsattar A., Ho M. Early infections in kidney, heart, and liver transplant recipients on cyclosporine. Transplantation. 1983 Sep;36(3):259–267. doi: 10.1097/00007890-198309000-00007. [DOI] [PubMed] [Google Scholar]
- Espevik T., Figari I. S., Shalaby M. R., Lackides G. A., Lewis G. D., Shepard H. M., Palladino M. A., Jr Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J Exp Med. 1987 Aug 1;166(2):571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janco R. L., English D. Cyclosporine and human neutrophil function. Transplantation. 1983 May;35(5):501–503. doi: 10.1097/00007890-198305000-00023. [DOI] [PubMed] [Google Scholar]
- Kahan B. D. Cyclosporine. N Engl J Med. 1989 Dec 21;321(25):1725–1738. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- Keown P. A. Emerging indications for the use of cyclosporin in organ transplantation and autoimmunity. Drugs. 1990 Sep;40(3):315–325. doi: 10.2165/00003495-199040030-00001. [DOI] [PubMed] [Google Scholar]
- Kharazmi A., Svenson M., Nielsen H., Birgens H. S. Effect of cyclosporin A on human neutrophil and monocyte function. Scand J Immunol. 1985 Jun;21(6):585–591. doi: 10.1111/j.1365-3083.1985.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Levitz S. M., Diamond R. D. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985 Nov;152(5):938–945. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- Levitz S. M., Selsted M. E., Ganz T., Lehrer R. I., Diamond R. D. In vitro killing of spores and hyphae of Aspergillus fumigatus and Rhizopus oryzae by rabbit neutrophil cationic peptides and bronchoalveolar macrophages. J Infect Dis. 1986 Sep;154(3):483–489. doi: 10.1093/infdis/154.3.483. [DOI] [PubMed] [Google Scholar]
- Lyman C. A., Simons E. R., Melnick D. A., Diamond R. D. Unopsonized Candida albicans hyphae stimulate a neutrophil respiratory burst and a cytosolic calcium flux without membrane depolarization. J Infect Dis. 1987 Nov;156(5):770–776. doi: 10.1093/infdis/156.5.770. [DOI] [PubMed] [Google Scholar]
- McIntosh L. C., Thompson A. W. Activity of the mononuclear phagocyte system in cyclosporin A-treated mice. Transplantation. 1980 Nov;30(5):384–386. doi: 10.1097/00007890-198011000-00017. [DOI] [PubMed] [Google Scholar]
- Roilides E., Mertins S., Eddy J., Walsh T. J., Pizzo P. A., Rubin M. Impairment of neutrophil chemotactic and bactericidal function in children infected with human immunodeficiency virus type 1 and partial reversal after in vitro exposure to granulocyte-macrophage colony-stimulating factor. J Pediatr. 1990 Oct;117(4):531–540. doi: 10.1016/s0022-3476(05)80684-5. [DOI] [PubMed] [Google Scholar]
- Roilides E., Uhlig K., Venzon D., Pizzo P. A., Walsh T. J. Enhancement of oxidative response and damage caused by human neutrophils to Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun. 1993 Apr;61(4):1185–1193. doi: 10.1128/iai.61.4.1185-1193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roilides E., Uhlig K., Venzon D., Pizzo P. A., Walsh T. J. Prevention of corticosteroid-induced suppression of human polymorphonuclear leukocyte-induced damage of Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun. 1993 Nov;61(11):4870–4877. doi: 10.1128/iai.61.11.4870-4877.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982 Mar;69(3):617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A. Therapeutic concentrations of glucocorticoids suppress the antimicrobial activity of human macrophages without impairing their responsiveness to gamma interferon. J Clin Invest. 1985 Nov;76(5):1755–1764. doi: 10.1172/JCI112166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. W., Moon D. K., Geczy C. L., Nelson D. S. Cyclosporin A inhibits lymphokine production but not the responses of macrophages to lymphokines. Immunology. 1983 Feb;48(2):291–299. [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli A., Cenci E., Marconi P., Rossi R., Riccardi C., Bistoni F. Immunosuppressive effect of cyclosporin A on resistance to systemic infection with Candida albicans. J Med Microbiol. 1989 Nov;30(3):183–192. doi: 10.1099/00222615-30-3-183. [DOI] [PubMed] [Google Scholar]
- Wajszczuk C. P., Dummer J. S., Ho M., Van Thiel D. H., Starzl T. E., Iwatsuki S., Shaw B., Jr Fungal infections in liver transplant recipients. Transplantation. 1985 Oct;40(4):347–353. doi: 10.1097/00007890-198510000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldorf A. R., Levitz S. M., Diamond R. D. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J Infect Dis. 1984 Nov;150(5):752–760. doi: 10.1093/infdis/150.5.752. [DOI] [PubMed] [Google Scholar]
- Weinbaum D. L., Kaplan S. S., Zdziarski U., Rinaldo C. R., Jr, Schroeder K. K. Human polymorphonuclear leukocyte interaction with cyclosporine A. Infect Immun. 1984 Mar;43(3):791–794. doi: 10.1128/iai.43.3.791-794.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisler R. L., Lindsey J. A., Proctor K. V., Morisaki N., Cornwell D. G. Characteristics of cyclosporine induction of increased prostaglandin levels from human peripheral blood monocytes. Transplantation. 1984 Oct;38(4):377–381. doi: 10.1097/00007890-198410000-00012. [DOI] [PubMed] [Google Scholar]


