Abstract
Neointimal hyperplasia is a critical component of restenosis, a major complication of angioplasty and related therapeutic procedures. We studied the effects of hyperlipidemia and the nonsteroidal anti-inflammatory drugs, aspirin (acetyl-salicylic acid; ASA), and sulindac, on neointimal formation in a mouse femoral arterial injury model. At 2 months of age, normolipidemic, wild-type (WT), and hyperlipidemic, apolipoprotein E-deficient (apoE−/−) mice were divided into three treatment groups: Western-type diet (WD), WD + ASA (200 mg/kg food), and WD + sulindac (300 mg/kg food). After 1 week, mice underwent arterial injury and treatments were maintained for 4 weeks. Histomorphometry of the injured arteries showed striking effects of plasma cholesterol levels and drug treatment on neointimal hyperplasia. In the WD or WD + ASA groups, apoE−/− mice had twice the neointimal area than WT mice (≈30,000 vs. 13,000 μm2 per section; P < 0.0001). Compared with ASA or WD alone, sulindac treatment resulted in ≈70% (P = 0.0001) and 50% (P = 0.01) reductions in the neointimal area in apoE−/− and WT mice, respectively. ASA, at a dose sufficient to inhibit platelet aggregation, did not affect neointimal formation in mice of either genotype. Evidence of macrophages was noted in the lesions of apoE−/− mice in the WD and WD + ASA groups, but remarkably, none was detectable with sulindac treatment, despite hyperlipidemia, suggesting early steps in the response to injury were abrogated. These results demonstrate sulindac reduces neointimal formation in both normolipidemic and hyperlipidemic settings and raise the possibility that similar benefits may be obtained in patients undergoing angioplasty and related procedures.
Keywords: atherosclerosis, restenosis
The incidence of restenosis after percutaneous revascularization techniques has remained high, ranging from 25% to 50% despite the introduction of stents and treatment with aspirin (acetyl-salicylic acid; ASA) and related antiplatelet compounds or anticoagulants (1, 2). Neointimal formation is a prominent feature of restenosis and involves the proliferation and migration of vascular smooth muscle cells (SMCs) and their elaboration of extracellular matrix after arterial injury (3–5).
Sulindac is a nonsteroidal anti-inflammatory drug (NSAID) that has antiproliferative and proapoptotic effects in vitro and in vivo. Notably, it has been shown to induce regression of colon polyps in patients with familial adenomatous polyposis and is used as a cancer chemopreventive agent for that disorder (6, 7). Given the response to arterial injury involves a rapid expansion of cell mass, we reasoned that if sulindac had similar effects in the vessel wall, it would limit neointimal formation after injury. To test this hypothesis, we used a mouse model of arterial injury reported recently (8).
Because the majority of patients who undergo angioplasty and related procedures have a history of hyperlipidemia (4, 9), our studies were performed in hyperlipidemic, apolipoprotein E-deficient (apoE−/−) mice, a widely used model of human atherosclerosis (10). ApoE−/− mice were pretreated for 1 week with Western-type diets (WD) containing either sulindac, ASA, or neither, and then subjected to transluminal femoral artery injury and maintained on their treatment regimens until they were killed 4 weeks later. A similar study was performed in normolipidemic [wild-type (WT)] mice. In both hyperlipidemic and normolipidemic mice, treatment with sulindac, but not ASA, resulted in a significant reduction in neointimal formation after injury. These results raise the exciting possibility that sulindac may be beneficial clinically as a therapeutic agent for the adverse consequences of percutaneous arterial interventions.
Materials and Methods
Animals and Injury Technique.
Male and female C57BL/6 mice, 12–14 weeks of age (weight = 33 ± 4 g) with apoE−/− (11, 12) or WT genotypes, were used. Breeding pairs of each genotype were purchased from The Jackson Laboratory. The colonies were expanded and studied at Mount Sinai School of Medicine, New York. All procedures were approved by the Institutional Animal Care and Use Committee, consistent with the Guide for the Care and Use of Laboratory Animals.
Mice underwent bilateral femoral artery endothelial denudation by passage of a 0.25-mm diameter angioplasty guidewire (Advanced Cardiovascular Systems, Temecula, CA). The wire was inserted into the femoral artery just distal to the inferior epigastric artery, and advanced and pulled back three times (8). At the time of arterial injury, the apoE−/− mice had not developed atherosclerotic lesions in the femoral artery (13).
Study Design.
Mice were maintained on a normal chow diet (11% wt/wt fat; PMI Nutrition International, Richmond, IN) until 1 week before injury, and then allocated to three different regimens: (i) WD, a diet containing 21% wt/wt fat (polyunsaturated/saturated ratio, 0.07), and 0.15% wt/wt cholesterol (Harlan Teklad, Madison, WI); (ii) WD containing ASA (WD + ASA; 200 mg/kg food; Sigma); or (iii) WD containing sulindac (WD + sulindac; sulindac sulfoxide; 300 mg/kg food; Sigma). The doses of ASA and sulindac were based on published studies in mice and rats (14, 15). ASA, commonly tried to prevent restenosis clinically, was used as a control for general NSAID effects.
After injury, mice were maintained on their treatment regimens for 4 weeks. Body weights were recorded at the times of injury and death. (Weight increases were similar among the groups, indicating comparable food consumption.)
Histopathological Assessments.
Histomorphometry was performed on arterial cross sections by two investigators, separately and in a blinded manner. (Those performing arterial injury also were blinded to animal genotype and treatment group.) To harvest specimens, mice were killed with an overdose of pentobarbital. The animal was perfusion-fixed with 4% paraformaldehyde in PBS at ≈100 mmHg. The perfusate was infused through a cannula (22 gauge; Angiocath, Becton Dickinson) inserted in the left ventricle; drainage was through an incision in the right atrium. The hind limbs were removed, fixed for 24 h, and decalcified overnight in 10% formic acid. Segments were cut transversely at 2-mm intervals at the level of arterial injury and paraffin embedded. Two 5-μm-thick sections of the midportion of each femoral artery were stained with combined Masson's trichome elastic (16).
Representative sections were stained for markers of macrophages [rat anti-mouse macrophages-monocytes (MOMA-2); Serotec; 2 μg/ml]; SMC (alkaline phosphatase-conjugated monoclonal anti-smooth muscle actin; 1:100; Sigma); and proliferation (Ki-67; rabbit polyclonal; NovoCastra, Newcastle, U.K.; 1:1,000). Sections were deparaffinized, blocked with 3% hydrogen peroxide, treated with 2% ovalbumin in PBS, and incubated with primary antibodies at 37°C for 2 h. Binding of the primary antibody was detected by using a biotinylated IgG (rat anti-mouse or goat anti-rabbit; 1:500; Vector Laboratories). After washing, sections were incubated with horseradish peroxidase-conjugated streptavidin for 30 min, developed with 3,3′-diaminobenzidine, and counterstained with hematoxylin. Negative controls were prepared by substitution of preimmune serum for the primary antibody. To assess apoptosis, sections were stained by using a modified terminal deoxynucleotidyltransferase-mediated UTP nick end labeling protocol as described (17). Severely (10 rads) and mildly (2 rads) irradiated thymuses were used as positive and negative controls, respectively.
For morphometric analysis, microscopic images were digitized and the amount of media and neointima was quantified by using the image-pro plus software (Media Cybernetics, Silver Spring, MD) as described (8). Areas are expressed as μm2 per section. The area occupied by macrophages was quantified as percentage of the intimal area with positive MOMA-2 staining. Presence of apoptosis and expression of Ki-67 were assessed at ×400 magnification by determining the percentage of neointimal cells with positive nuclear reactivity (brown staining).
Plasma Lipid Analysis.
Plasma total cholesterol levels were measured in venous blood samples by an enzymatic immunoassay (Boehringer Mannheim) at the time of treatment-group randomization and the time of death.
Platelet Aggregation Studies.
To confirm that ASA treatment exerted systemic effects on platelet function, aggregation studies were performed on platelets freshly isolated from apoE−/− mice from the WD alone and WD + ASA groups. Blood was collected in citrate by retroorbital bleeding (500 μl) and platelet-rich plasma was obtained by centrifugation (800 rpm, 6 min). Because ASA inhibits platelet aggregation by inhibiting cyclooxygenase (COX), the prostaglandin precursor arachidonic acid (final concentration, 0.5 mM) was used to stimulate aggregation. Reagents and aggregometer were purchased from Chrono-Log (Havertown, PA) and used as described in the manufacturer's protocol.
Statistical Analysis.
Numerical data are reported as means ± SEM. P values of < 0.05 were considered significant. One- and two-way ANOVA, Bonferroni's test for multiple comparisons, and unpaired t testing were performed as appropriate, by using the graphpad prizm software package (GraphPad, San Diego, CA). For one-way ANOVA, the factor under consideration was treatment group; for two-way, factors were treatment group and genotype. All data initially were analyzed for the effect of gender; because there were no significant differences, final analyses include pooled data from males and females.
Results
Plasma Cholesterol Levels.
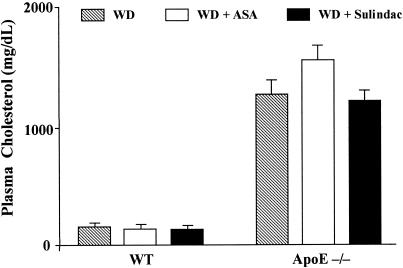
For each genotype, mean plasma cholesterol levels were comparable among the treatment groups at the time of randomization (data not shown). Levels at the time of death are summarized in Fig. 1. There was a significant difference between mean levels of WT and apoE−/− mice (P < 0.0001; two-way ANOVA), but no significant differences related to the treatments (P > 0.05).
Figure 1.
Plasma cholesterol levels 4 weeks after mouse femoral artery injury. ApoE−/− and WT mice were fed WD, WD + ASA, or WD + sulindac, 1 week before and 4 weeks after arterial injury. Venous blood samples were taken for cholesterol analysis. Bars represent means ± SEM based on results from 9–14 mice per group.
Histomorphometric Changes.
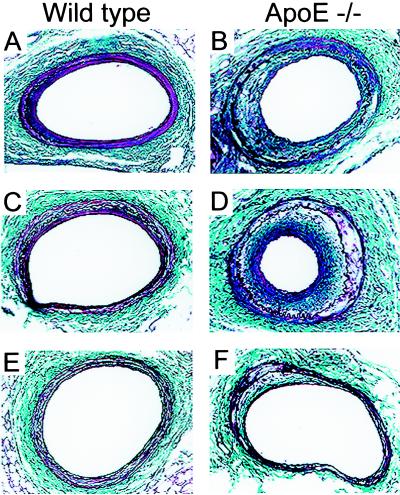
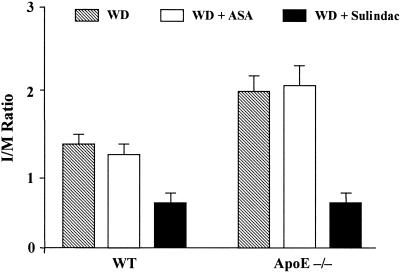
Four weeks after injury, neointimal formation was apparent in femoral arteries from both WT and apoE−/− mice (Fig. 2). Summaries of the morphometric parameters related to the media and intima and their statistical analyses are given in Fig. 3 and Table 1. For both genotypes, sulindac treatment had a major effect on the amount of neointima formed compared with the two other treatment groups. This was most evident in sulindac-treated apoE−/− mice (Fig. 2), in which the neointima [expressed as either the intimal area or the intima-to-media (I/M) ratio] was only ≈30% of that formed in the WD or WD + ASA groups (Table 1; Fig. 3).
Figure 2.
Histologic appearances of mouse femoral arteries 4 weeks after injury. Representative photomicrographs (×200; combined Masson-elastic stain) of lesions from mice in each treatment group are displayed. (A and B) WD alone. (C and D) WD + ASA. (E and F) WD + sulindac. In B and D, the looser packing of cells and the many lucent foci (“clefts”) are characteristic of increased deposition of extracellular matrix and cholesterol, respectively.
Figure 3.
Effects of genotype and drug treatments on the I/M ratios of lesions from injured mouse femoral arteries. Four weeks after injury, femoral arterial sections were taken for morphometric analysis and the intimal and medial areas were determined. The ratio of these areas (I/M ratio) was calculated for each section. The means ± SEM (n = 9–14 for each group) are displayed.
Table 1.
Summary of data and statistical analyses
| Morphometric data
|
||||||
|---|---|---|---|---|---|---|
| WT
|
ApoE−/−
|
|||||
| WD (n = 10) | WD + ASA (n = 10) | WD + SUL (n = 10) | WD (n = 14) | WD + ASA (n = 9) | WD + SUL (n = 14) | |
| Intimal area | 12,790 ± 1,938 | 12,810 ± 1,666 | 6,233 ± 1,222 | 30,980 ± 3,647 | 29,720 ± 6,203 | 8,222 ± 1,754 |
| Medial area | 9,916 ± 1,363 | 10,080 ± 695 | 10,260 ± 627 | 15,790 ± 1,427 | 13,640 ± 1,239 | 12,130 ± 575 |
| I/M ratio | 1.34 ± 0.1 | 1.26 ± 0.1 | 0.62 ± 0.1 | 1.96 ± 0.2 | 2.04 ± 0.3 | 0.67 ± 0.1 |
| Dependent variable | Two-way ANOVA
|
|
|---|---|---|
| Effect of genotype; WT, apoE−/− | Effect of treatment; WD, WD + ASA, WD + SUL | |
| Medial area | P < 0.0001 | NS |
| Intimal area | P < 0.0001 | P < 0.0001 |
| I/M ratio | P < 0.0012 | P < 0.0001 |
| Genotype | One-way ANOVA
|
||
|---|---|---|---|
| Dependent variable | Overall P value for treatment group differences | Significantly different groups, P < 0.05 | |
| WT | Medial area | NS | None |
| WT | Intimal area | P = 0.0108 | SUL vs. WD; SUL vs. ASA |
| WT | I/M ratio | P = 0.0009 | SUL vs. WD; SUL vs. ASA |
| ApoE−/− | Medial area | NS | None |
| ApoE−/− | Intimal area | P = 0.0001 | SUL vs. WD; SUL vs. ASA |
| ApoE−/− | I/M ratio | P = 0.0001 | SUL vs. WD; SUL vs. ASA |
WT or apoE−/− mice maintained on chow diets were divided into three treatment groups 1 week before arterial injury: WD, WD + ASA (ASA in the one-way ANOVA summary), or WD + sulindac (SUL in the one-way ANOVA summary). Four weeks after injury, sections of the femoral artery were taken for morphometric analysis. The values under Morphometric data are mean areas (μm2 per lesion section) ± SEM, or for the I/M ratio, the mean ± SEM of the intimal area divided by the medial area. Two-way ANOVA was used to determine statistically significant effects of genotype and treatment group status on the indicated dependent variables. For each genotype, one-way ANOVA with Bonferroni's Multiple Comparison Test was used to compare means among the treatment groups (one-way ANOVA). NS, not significant (P > 0.05).
Statistical testing of the intimal area and I/M ratio data disclosed a highly significant (P < 0.0001) treatment effect, but also showed statistically significant differences related to genotype, i.e., whether a mouse was WT or apoE−/− (P < 0.001). Compared with WT, apoE−/− mice also had a significant increase in the medial area (P < 0.0001), independent of treatment (Table 1).
To further analyze differences related to treatment, statistical comparisons were performed separately for WT and apoE−/− mice. Results of these analyses were identical: for either genotype, the WD + sulindac group had significantly lower mean values for intimal area (P < 0.01) and I/M ratio (P < 0.001) compared with the corresponding values in the WD or WD + ASA groups. In contrast, for either genotype, compared with treatment with the WD alone, ASA had no significant effects on either intimal area or the I/M ratio (Table 1; Fig. 3). Interestingly, although I/M ratios for the WD (1.96 ± 0.2) and WD + ASA (2.04 ± 0.3) groups of apoE−/− mice were higher than the corresponding values in the groups of WT mice (1.34 ± 0.1 and 1.26 ± 0.1, respectively), the I/M ratios of the two different WD + sulindac groups were statistically comparable [0.67 ± 0.1 (apoE−/−) vs. 0.62 ± 0.1 (WT); P > 0.05).
Systemic Effects of ASA.
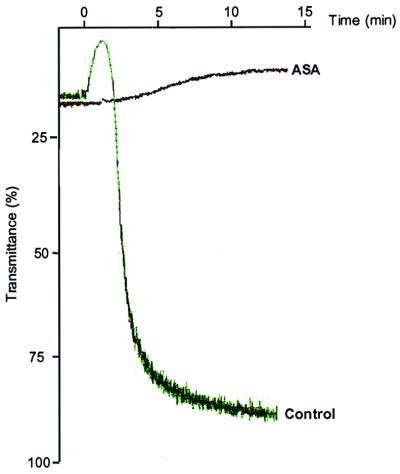
A number of explanations for the lack of effect of ASA on neointimal formation were considered. Hypolipidemia or toxic effects were excluded based on the results for plasma cholesterol levels (Fig. 1) and weight gain (Materials and Methods), respectively. To test whether a dose of ASA adequate to be effective systemically had been given, platelet aggregation studies were performed on blood obtained from apoE−/− mice fed the WD or WD + ASA for 1 week (i.e., length of treatment before injury). Fig. 4 shows assays from representative samples. Note that in the mouse given the ASA-containing diet, platelet aggregation was completely suppressed, demonstrating that the dose of ASA was adequate to produce systemic effects. These results suggest that sulindac exerts effects, perhaps related to its specificity or potency, that are not common to all NSAID.
Figure 4.
Aggregation of platelets from apoE−/− mice. Before isolating platelet-rich plasma, mice were fed a WD with ASA or without (control) for 1 week. Arachidonic acid was added and the formation of aggregates was detected by an increase in light transmittance over time. Representative assays are shown.
Effects of Sulindac on Macrophage Content, Cellular Proliferation, and Apoptosis.
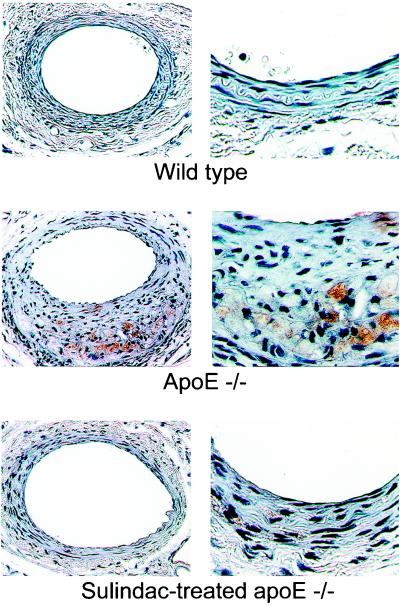
Based on immunostaining for the macrophage marker, MOMA-2, lesions from apoE−/− mice on WD alone [and WD + ASA (not shown)] contained macrophages, but lesions from WT mice on WD alone did not (Fig. 5). Note the location of macrophages in the neointima from the apoE−/− mouse (Fig. 5 Middle) is not periluminal, but near the internal elastic lamina, which may reflect macrophage infiltration and adhesion shortly after endothelial denudation and before migration of SMC toward the lumen. For both WT and apoE−/− mice in either the WD or WD + ASA group, analysis of the SMC marker, α-actin, revealed SMC throughout the neointima and media (data not shown).
Figure 5.
Macrophage content of lesions from mouse femoral arteries 4 weeks after injury. Arterial sections were immunostained for the macrophage marker, MOMA-2, whose presence is indicated by a brown color. Note the substantial macrophage infiltration and cholesterol clefts (lucent foci) adjacent to the internal elastic lamina in the section from the apoE−/− mouse from the WD group (Middle). Original magnification: Left, ×200; and Right, ×630.
In contrast to the results for apoE−/− mice in the WD group, there was a remarkable absence of MOMA-2 immunostaining in lesions from apoE−/− mice treated with sulindac (Fig. 5 Bottom). Of five arteries examined, staining for MOMA-2 was found only at a trace level in the lesion of a single mouse (Fig. 5 Bottom). The quantitative analysis of the percentage of intimal area stained for MOMA-2 revealed that for apoE−/− mice (n = 8) on WD alone, the average value was 8.4 ± 2.9, significantly different (P < 0.01) from the value of 0.02 ± 0.02 in lesions from apoE−/− mice (n = 5) in the WD + sulindac group. Consistent with the paucity of macrophages in the lesions of sulindac-treated apoE−/− mice was the absence of cholesterol “clefts” (reflecting lipid accumulation associated with foam cell formation). In contrast, clefts are present in macrophage-containing lesions from apoE−/− mice not treated with sulindac (e.g., note lucent foci [clefts] intermingled with immunostained area in the section shown in Fig. 5 Middle, and compare with the appearance of the section in the Bottom. Lucent foci also are apparent in Fig. 2 B and D).
Given the marked reduction in neointimal formation associated with sulindac treatment, cell proliferation and apoptosis were assessed in the intimal lesions from apoE−/− mice in the WD and WD + sulindac groups. There were no significant differences in either the percentage of cells stained for Ki-67 (WD, 1.1 ± 0.5%; WD + sulindac, 1.7 ± 0.6%; P > 0.05), or positive for terminal deoxynucleotidyltransferase-mediated UTP end labeling (1.0 ± 0.5% vs. 1.2 ± 1%; P > 0.05).
Discussion
Restenosis resulting from neointimal formation after angioplasty and related procedures remains a major clinical problem in the management of coronary artery disease (18, 19). Major limitations of previous animal models used to study restenosis have included their relatively large size or the inability to study effects of hypercholesterolemia (e.g., refs. 20–22). To overcome these limitations, we recently established a reproducible femoral arterial injury model in mice that results in pathology similar to that observed in clinical restenosis (8). By using both WT and apoE−/− mice, we were able to determine the separate effects of hyperlipidemia and drug treatment on neointimal formation after arterial injury.
There were two major findings in the present study. First, mice in the WD and WD + ASA groups had more than 2-fold increases in neointimal formation associated with hyperlipidemia. This is clinically relevant because the majority of patients undergoing vascular interventions have hyperlipidemia (4, 9). The quantitative difference was associated with qualitative changes in the lesions; intimal lesions from the hyperlipidemic apoE−/− mice were loosely arranged (perhaps indicating more extracellular matrix, ref. 22), and contained abundant macrophages (based on immunostaining) and cholesterol clefts adjacent to the media. SMCs were primarily periluminal, although also present deeper into the lesion. In contrast, lesions in WT mice were essentially devoid of macrophages and contained SMCs throughout.
The second major finding was that sulindac treatment resulted in a marked decrease (up to 70%) in neointimal formation after injury in both apoE−/− and WT mice. Therefore, sulindac treatment had marked efficacy in both hyperlipidemic and normolipidemic settings, limiting the I/M ratio to less than 0.7 in both genotypes. In addition to the quantitative changes, sulindac treatment abolished macrophage infiltration and cholesterol deposition in the neointima of apoE−/− mice, despite extremely elevated plasma cholesterol levels. Notably, the other NSAID, ASA, had neither the quantitative nor the qualitative effects of sulindac, despite plasma levels sufficient to exert systemic effects, as demonstrated by inhibition of platelet aggregation. These results suggest that sulindac has specific effects on macrophage-related processes. This is especially intriguing because macrophage infiltration appears to be a crucial determinant of restenosis in humans. For example, in atherectomy specimens obtained after balloon angioplasty, the degree of infiltration of macrophages was a strong predictor of clinical restenosis and subsequent cardiovascular events (23).
Effects of sulindac on macrophages directly (e.g., by its known ability to inhibit NF-κB activation, ref. 24) or indirectly (e.g., by possibly inhibiting chemoattractant or adhesion molecule expression by SMCs and endothelial cells) would, therefore, be expected to have a substantial impact on the response to arterial injury. Evidence from the present study supports the modulation of macrophage-related processes by sulindac. As noted, in apoE−/− mice having comparable levels of hyperlipidemia (a factor well known to promote macrophage infiltration and prolong their retention in the arterial wall, ref. 3), macrophages and cholesterol deposits were absent in lesions of sulindac-treated mice and abundant in the nonsulindac treated. Because monocyte/macrophage cells secrete factors that can stimulate SMC proliferation and migration, if sulindac limited their interactions with or within the injured arterial wall, SMC “activation” could have been impaired. Based on the location of the macrophages (near the internal elastic lamina, Fig. 5) and the similar I/M ratios in sulindac-treated WT and apoE−/− mice, macrophage interactions inducing neointimal formation probably occurred early after injury. Such interactions, which appear to be transient in the normolipidemic setting (8), could be prolonged in hyperlipidemia, leading to stronger activation of SMC and increased neointimal formation, consistent with our observations.
Sulindac may have decreased intimal lesions by several other mechanisms. One possibility is COX inhibition. Sulindac has two major metabolites, a sulfide and a sulfone (25). Only the sulfide inhibits COX activity, and of the two isoforms, COX-1 and COX-2, the former is more affected. Because ASA, a nonselective COX inhibitor, was ineffective in limiting neointimal formation, inhibition of eicosanoid synthesis by blocking COX catalytic activity is unlikely to be the basis for the efficacy of sulindac in the present study.
Given the prominent role of SMC in neointimal formation (3, 26), another possibility is that sulindac inhibited proliferation and promoted apoptosis of these cells in the vessel wall, as it has been shown to do in certain types of cells in culture (27–29) and in vivo (7, 30). An attractive feature of this potential mechanism is that these effects can be independent of COX activity (27, 31), consistent with the ineffectiveness of ASA. Sulindac treatment, however, did not affect results of our assays for proliferation or apoptosis, although this may have been caused by sampling at 4 weeks after injury, by which time the rapid cell-mass expansion is typically over (26).
Still other potential molecular mechanisms for the effects of sulindac on neointimal proliferation may involve its ability to inhibit DNA binding of the peroxisome proliferator-activated receptor-delta transcription factor (32), cGMP-dependent phosphodiesterase-5 activity,‡‡ and Ras signaling (34), or to increase the intracellular formation of ceramide, a known inducer of apoptosis (35).
Because the pathogenesis of neointimal formation after arterial injury depends heavily on the turnover of SMC, possible effects of sulindac on cell proliferation and apoptosis have been emphasized. This does not preclude other effects on SMC. For example, sulindac or its metabolites may inhibit the migration of SMC, their elaboration of extracellular matrix (consistent with the denser cellularity of the lesions from apoE−/− mice treated with sulindac compared with those receiving WD + ASA or WD alone (Figs. 2 and 5), or their expression of adhesion molecules. Sulindac also may affect other cell types in the vessel wall. (The macrophage as a target was discussed above.) Endothelial cells may be a target of sulindac, because NSAID have been shown to interfere with the effects of angiogenic factors on tube formation in cultures of these cells (36). Effects on endothelial cells could be important because of the crucial role of these cells in arterial remodeling, known to involve molecular “crosstalk” between endothelium and SMC (37), and the reports that treatment with vascular endothelial growth factor may limit neointimal formation (19).
The clinical relevance of our findings is supported by the following considerations. We used a dose of sulindac that is significantly lower than the maximum nontoxic dose for rodents (14, 15). Compared with a typical clinical dose (≈6 mg/kg per day) for arthritis treatment or chemoprevention in familial adenomatous polyposis, the mouse dose is significantly higher (≈25 mg/kg mouse); however, the expected plasma level in the mouse (38) is less than twice that of humans (33). Thus, very high plasma levels of sulindac may not be required for clinical efficacy in restenosis. In addition, potential for toxicity may be limited because of the short-term treatment, in contrast to the chronic administration for other disorders.
In summary, our results demonstrate that hyperlipidemia has a major influence on neointimal formation after arterial injury in mice. Sulindac, which has proven therapeutic effects in patients with familial adenomatous polyposis, significantly reduced neointimal formation after injury in both hyperlipidemic and normolipidemic mice. Another NSAID, ASA, was ineffective, despite achieving levels sufficient to produce inhibitory effects on platelet aggregation. We therefore postulate that sulindac may have specific and beneficial effects on the arterial wall that may make it a useful clinical agent for the prevention of vascular restenosis after angioplasty and related procedures.
Acknowledgments
We thank Dr. Barry Coller for help in the platelet aggregation studies; Marija Drobnjak, Veronica Gulle, and Maria Socorro Jiao for performing histological, immunohistochemical, and terminal deoxynucleotidyltransferase-mediated UTP end labeling assays; and Dr. Newsha Ghodsi (supported by National Institutes of Health Training Grant T32 HL07824), for performing some of the arterial injury procedures and morphometric analyses. This work was supported in part by a grant-in-aid from the Spanish Heart Association to M.R., and funds from the Zena and Michael A. Wiener Cardiovascular Institute and the National Institutes of Health (HL61814) to E.A.F., and the Arthur and Rochelle Belfer Foundation to S.J.S.
Abbreviations
- apoE−/−
apolipoprotein E-deficient
- ASA
acetyl-salicylic acid (aspirin)
- COX
cyclooxygenase
- I/M
intima-to-media
- NSAID
nonsteroidal anti-inflammatory drug
- SMC
smooth muscle cell
- WD
Western-type diet
- WT
wild type
- MOMA-2
rat anti-mouse macrophages-monocytes
Footnotes
See commentary on page 12400.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.210394497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.210394497
Piazza, G., Li, H., Liu, L., Sperl, G., Pamukcu, R., Thompson, W. J. & Ahnen, D. J. (1999) Gastroenterology 116, 485 (abstr.).
References
- 1.Bittl J A. N Engl J Med. 1996;24:1290–1302. doi: 10.1056/NEJM199610243351707. [DOI] [PubMed] [Google Scholar]
- 2.Knight C J, Curzen N P, Groves P H, Patel D J, Goodall A H, Wright C, Clarke D, Oldershaw P J, Fox K M. Eur Heart J. 1999;20:1783–1790. doi: 10.1053/euhj.1999.1545. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 4.Popma J, Califf R, Topol E. Circulation. 1991;84:1426–1436. doi: 10.1161/01.cir.84.3.1426. [DOI] [PubMed] [Google Scholar]
- 5.Liu M W, Roubin G S, King S B., III Circulation. 1989;79:1374–1387. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- 6.Giardiello F M, Hamilton S R, Krush A J, Piantadosi S, Hylind L M, Celano P, Booker S V, Robinson C R, Offerhaus G J. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 7.Shiff S J, Rigas B. Gastroenterology. 1997;113:1992–1998. doi: 10.1016/s0016-5085(97)99999-6. [DOI] [PubMed] [Google Scholar]
- 8.Roque M, Fallon J T, Badimon J J, Zhang W X, Taubman M B, Reis E D. Arterioscler Thromb Vasc Biol. 2000;20:335–342. doi: 10.1161/01.atv.20.2.335. [DOI] [PubMed] [Google Scholar]
- 9.Boccuzzi S J, Weintraub W S, Kosinski A S, Roehm J B, Klein J L. Am J Cardiol. 1998;81:632–636. doi: 10.1016/s0002-9149(97)00980-6. [DOI] [PubMed] [Google Scholar]
- 10.Osada J, Joven J, Maeda N. Curr Opin Lipidol. 2000;11:25–29. doi: 10.1097/00041433-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S H, Reddick R L, Piedrahita J A, Maeda N. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 12.Plump A S, Smith J D, Hayek T, Aalto-Setala K, Walsh A, Verstuyft J G, Rubin E M, Breslow J L. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima Y, Plump A S, Raines E W, Breslow J L, Ross R. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 14.Rao K V, Detrisac C J, Steele V E, Hawk E T, Kelloff G J, McCormick D L. Carcinogenesis. 1996;17:1435–1438. doi: 10.1093/carcin/17.7.1435. [DOI] [PubMed] [Google Scholar]
- 15.Duperron C, Castonguay A. Carcinogenesis. 1997;18:1001–1006. doi: 10.1093/carcin/18.5.1001. [DOI] [PubMed] [Google Scholar]
- 16.Garvey W. Stain Technol. 1984;59:213–214. doi: 10.3109/10520298409113858. [DOI] [PubMed] [Google Scholar]
- 17.Gavrieli Y, Sherman Y, Ben-Sasson S A. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virmani R, Farb A. Curr Opin Lipidol. 1999;10:499–506. doi: 10.1097/00041433-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Isner J M, Walsh K, Rosenfield K, Schainfeld R, Asahara T, Hogan K, Pieczek A. Hum Gene Ther. 1996;7:989–1011. doi: 10.1089/hum.1996.7.8-989. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz R S. Lab Invest. 1994;71:789–791. [PubMed] [Google Scholar]
- 21.Geary R L, Hohler T R, Vergel S, Kirkman T R, Clowes A W. Circ Res. 1994;74:14–23. doi: 10.1161/01.res.74.1.14. [DOI] [PubMed] [Google Scholar]
- 22.Clowes A W, Reidy M A, Clowes M M. Lab Invest. 1986;54:295–303. [PubMed] [Google Scholar]
- 23.Moreno P R, Bernardi V H, Lopez-Cuellar J, Newell J B, McMellon C, Gold H K, Palacios I F, Fuster V, Fallon J T. Circulation. 1996;94:3098–3102. doi: 10.1161/01.cir.94.12.3098. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto Y, Yin M J, Lin K M, Gaynor R B. J Biol Chem. 1999;274:27307–27314. doi: 10.1074/jbc.274.38.27307. [DOI] [PubMed] [Google Scholar]
- 25.Insel P. In: Goodman and Gilman's: The Pharmacological Basis of Therapeutics. Hardman J G, Limbird L E, Molinoff P B, Ruddon R W, Gilman A G, editors. New York: McGraw–Hill; 1996. pp. 617–657. [Google Scholar]
- 26.Braun-Dullaeus R C, Mann M J, Dzau V J. Circulation. 1998;98:82–89. doi: 10.1161/01.cir.98.1.82. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Morham S G, Langenbach R, Young D A. J Exp Med. 1999;190:451–459. doi: 10.1084/jem.190.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim J T, Piazza G A, Han E K, Delohery T M, Li H, Finn T S, Buttyan R, Yamamoto H, Sperl G J, Brendel K, et al. Biochem Pharmacol. 1999;58:1097–1107. doi: 10.1016/s0006-2952(99)00200-2. [DOI] [PubMed] [Google Scholar]
- 29.Shiff S J, Qiao L, Tsai L-L, Rigas B. J Clin Invest. 1995;96:491–503. doi: 10.1172/JCI118060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiu C H, McEntee M F, Whelan J. Cancer Res. 1997;57:4267–4273. [PubMed] [Google Scholar]
- 31.Hanif R, Pittas A, Feng Y, Koutsos M I, Qiao L, Staiano-Coico L, Shiff S I, Rigas B. Biochem Pharmacol. 1996;52:237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- 32.He T C, Chan T A, Vogelstein B, Kinzler K W. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duggan D E, Hare L E, Ditzler C A, Lei B W, Kwan K C. Clin Pharmacol Ther. 1977;21:326–335. doi: 10.1002/cpt1977213326. [DOI] [PubMed] [Google Scholar]
- 34.Shiff S J, Rigas B. J Exp Med. 1999;190:445–450. doi: 10.1084/jem.190.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan T A, Morin P J, Vogelstein B, Kinzler K W. Proc Natl Acad Sci USA. 1998;95:681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones M K, Wang H, Peskar B M, Levin E, Itani R M, Sarfeh I J, Tarnawski A S. Nat Med. 1999;5:1418–1423. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- 37.Yancopoulos G D, Klagsbrun M, Folkman J. Cell. 1998;93:661–664. doi: 10.1016/s0092-8674(00)81426-9. [DOI] [PubMed] [Google Scholar]
- 38.Oshima M, Dinchuk J E, Kargman S, Oshima H, Hancock B, Kwong E, Trzaskos J M, Evans J F, Taketo M M. Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]