Abstract
Aims
Vanadium is currently undergoing clinical trials as an oral drug in patients with noninsulin-dependent diabetes mellitus. Furthermore, vanadium occurs in elevated concentrations in the blood of patients receiving intravenous albumin solutions containing large amounts of the metal ion as an impurity. The present study was performed to examine the pharmacokinetics of vanadium in humans following a single intravenous (i.v.) dose of a commercial albumin solution containing a high amount of vanadium.
Methods
The study was conducted in five healthy volunteer subjects who received intravenously 90 ml of a commercial 20% albumin infusion solution containing 47.6 µg vanadium as an impurity. Vanadium concentrations in serum and urine were determined by electrothermal atomic absorption spectrometry.
Results
Vanadium serum concentrations after i.v. administration were measured for 31 days. The data could be fitted by a triexponential function corresponding formally to a three-compartment model. There was an initial rapid decrease in serum concentrations with half-lives of 1.2 and 26 h. This was followed by a long-terminal half-life time of 10 days. The terminal phase accounted for about 80% of the total area under the serum concentration-time curve (AUC). The mean apparent volume of distribution of the central compartment was found to be 10 l. The volume of distribution at steady state was 54 l, and total clearance was 0.15 l h−1. Vanadium was mainly excreted by the kidneys. About 52% of the dose was recovered in the urine after 12 days.
Conclusions
This study provides data on vanadium pharmacokinetics in healthy humans.
Keywords: albumin, electrothermal atomic absorption spectrometry (ETAAS), half-life, parenteral infusion therapy, pharmacokinetics, ultra trace metal analysis, vanadium
Introduction
Vanadium is an ultratrace element occurring in most mammalian cells and is assumed to be essential [1, 2]. The main source of vanadium intake is food. The acute and chronic toxic effects of this element when absorbed in greater amounts, usually by the respiratory tract, are well documented [3, 4]. The pharmacological and physiological actions of vanadium have been investigated widely over the last 20 years [5–7]. Of special interest are the insulin-like properties of vanadium. Although this action has been known since the end of the 19th century, when it was used as a panacea for various diseases [4], its real therapeutic potential has only recently become clear. Short-term clinical trials with vanadium have been performed in type II (noninsulin-dependent) diabetic patients [8–10], and the results suggest that vanadium may have a potential role in the adjunctive therapy of these patients [11].
In addition to the intended therapeutic use of vanadium, its accidental administration can occur because it is present as a contaminant in infusion solutions. In the course of our investigations [12], we were surprised to discover elevated vanadium concentrations (0.15–0.4 µg l−1) in patients who underwent cardiac surgery compared with healthy blood donors (0.023–0.108 µg l−1). The reason was that patients were receiving intravenous albumin solutions containing vanadium at concentrations ranging from 1.1 to 677 µg l−1.
In order to investigate the physiological implications of such contamination, it is necessary to understand the pharmacokinetics of this metal. Detailed studies of intravenously administered vanadium have been carried out only in animals [13–19]. The absence of human data was mainly due to the lack of a selective, reliable and sensitive analytical technique for the determination of vanadium in blood.
Methods
Subjects
The study was performed in five male healthy nonsmoking volunteers with an average age of 27 years (23–39 years) and an average weight of 86 kg (72–105 kg). Prior to the study the volunteers underwent a physical examination that included routine laboratory tests. No abnormalities, especially with respect to liver and kidney function, were observed. The volunteers took no medication for at least 3 weeks before and during the study. The study was approved by the ethics committee of the Ludwig-Maximilians-University of Munich. The volunteers were informed of the potential hazards verbally and in writing before they gave their written consent.
Albumin solution
The commercially available human albumin solution (Albumin 20% human™ for i.v. infusions), which is heavily contaminated with vanadium, was obtained from Octapharma, Langenfeld, Germany. The total vanadium concentration was 528.8 µg l−1 as determined by electrothermal atomic absorption spectrometry. The electron paramagnetic resonance spectroscopy revealed that> 80% of the vanadium was in the +5 oxidation state, i.e. in the vanadate form.
Protocol
Human albumin solution (90 ml) corresponding to a dose of 47.6 µg vanadium was infused intravenously over 20 min to each volunteer starting at 08.00 h. An indwelling catheter used for sample collection during the first phase of 80 min was placed in an antecubital vein of the contralateral arm. Blood samples (10 ml each) were collected 15 min prior to vanadium administration, 10 min after starting the infusion, and 1, 10, 30, 60 min, 3, 6, 12, 24, 72 h after the end of infusion followed by collection every fourth day for up to 31 days. The first urine sample was collected during the 24-h period prior to vanadium administration. Thereafter urine sampling continued as follows: 8–10, 10–12, 14–16, 16–20, 20–24 h on the first day, 24–8, 8–16, 16–24 h on the second day and 24–8, 8–16, 16–8 h on the third day. From day 4 to day 12, 24-h urine samples were collected. Blood was centrifuged and serum and urine samples were kept frozen at −40 °C until they were analysed.
Analytical methods
Total vanadium was determined by electrothermal atomic absorption spectrometry (ETAAS) as described previously [12]. The limit of detection (LOD) of vanadium was 11 ng l−1, the limit of quantification (LOQ) 17 ng l−1 and the coefficient of variation was 5.5% at 1.54 µg l−1. Electron paramagnetic resonance (EPR) spectra were measured on an Elecsys 580 EPR spectrometer (Bruker, Rheinstetten, Germany).
Data analysis
Polyexponential equations were fitted to the time course of serum concentrations and to the amounts excreted in urine by nonlinear regression analysis using Sigmaplot™ software (Jandel Scientific, Erkrath, Germany). The fitting of the serum concentration (C)-time curve was performed using weighted least squares by defining the weighting variable w = 1/C2. Calculations of the pharmacokinetic parameters were performed applying common equations as published in the literature [20, 21]. Names and symbols of the pharmacokinetic parameters were chosen in accordance with the literature [22].
Results and discussion
Time course of vanadium in serum
The mean vanadium serum concentration-time curve following single intravenous administration of 47.6 µg vanadium declined rapidly during the first few hours. After 24 h the serum concentration had dropped to less than 30% of its peak value immediately after the end of the infusion (Figure 1). Subsequently, serum vanadium concentration decreased more slowly, approaching the value at time zero (before start of infusion) after 31 days. As assessed by a plot of residuals and the correlation coefficient (r = 0.998) the serum concentrations (C) could quite well be fitted by a three-exponential equation of the type C = C1·e-l1·t + C2·e-l2·t + Cz·e-lz·t that formally corresponds to a three-compartment model. The pertinent values of the intercepts (C1, C2, Cz), rate constants (λ1, λ2, λz) and the derived pharmacokinetic parameters are given in Table 1. The mean half-lives (t1/2) for the rapid, intermediate, and slow phase of the concentration-time profile were 1 h, 26 h and 10 days, respectively.
Figure 1.
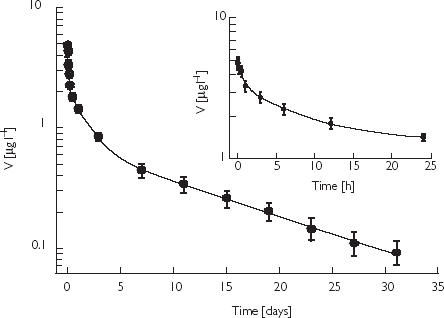
Mean serum concentration-time curve of vanadium in five healthy volunteers treated with 90 ml of an albumin solution containing 47.6 µg vanadium, V vanadium serum concentration, d (h) days (hours) after dosing. The rapid decline of the vanadium concentration during 24 h after infusion is shown as an inset. Points are mean values ± SEM.
Table 1.
Mean pharmacokinetic parameters of vanadium in serum from five healthy male volunteers after intravenous administration of 47.6 µg. The serum concentrations were determined for 31 days. A three exponential equation was fitted to the serum concentrations by means of nonlinear regression. Standard symbols [22]
| Parameter | Value ± SEM | |
|---|---|---|
| Intercept of fast disposition slope with ordinate | [C1 (µg l−1)] | 2.41 ± 0.22 |
| Intercept of intermediate disposition slope with ordinate | [C2 (µg l−1)] | 1.62 ± 0.15 |
| Intercept of slow disposition slope with ordinate | [Cz (µg l−1)] | 0.69 ± 0.05 |
| Fast disposition rate constant | [λ1 (h−1)] | 0.57 ± 0.14 |
| Intermediate disposition rate constant | [λ2 (h−1)] | 0.027 ± 0.004 |
| Slow disposition rate constant | [λz (h−1)] | 0.0028 ± 0.001 |
| Elimination half-life associated with fast disposition phase | [t1/2,1 (h)] | 1.2 |
| Elimination half-life associated with intermediate disposition phase | [t1/2,2 (h)] | 25.7 |
| Elimination half-life associated with terminal disposition phase | [t1/2,z (days)] | 10.4 |
| Volume of central compartment | [Vc (l)] | 10.1 |
| Volume of distribution at steady state | [Vss (l)] | 54.0 |
| Volume of distribution during terminal phase | [Vz (l)] | 55.0 |
| Total area under serum concentration-time curve | [AUC (µg l−1 h)] | 311.5 |
| Total body clearance from serum | [CL (l h−1)] | 0.153 |
In order to assess the contribution of the three phases to the total area under the curve (AUC) the partial AUCs (Ci λi−1) were calculated. The terminal phase contributed 79.2% to total AUC, the rapid and intermediate phases only 1.6 and 19.2%. Thus, the long terminal half-life is the dominant phase that determines the cumulative behaviour of vanadium. Furthermore, it becomes evident that the serum concentrations of vanadium have to be measured over extended time periods in order to describe its pharmacokinetics adequately.
The apparent volume of distribution (Table 1) of vanadium in our study was of the order of 50 l (about 0.6 l kg−1). However, only total concentrations in serum were determined and vanadium is bound to serum proteins especially to transferrin. Thus the calculated volume of distribution suggests that vanadium exhibits appreciable tissue binding. This view is supported by animal studies indicating that vanadium accumulates in tissues such as liver, kidneys, bone, and spleen [15, 23–25].
Vanadate is reported to be weakly bound to albumin [26–28]. We found that it is completely dialysable from albumin solutions (unpublished results). Thus, i.v. administered commercial albumin solutions with high vanadium content can be expected to represent pools of easily disposable vanadium. In the circulating blood vanadium is supposed to exist in two oxidation states, i.e. vanadyl and vanadate. The fractions of the two species depend on the oxygen tension and the presence of reducing/oxidizing substances [27, 29, 30]. However, both species bind predominantly to transferrin [26, 27, 31–33].
The general pattern of vanadium kinetics observed in our study in humans is remarkably similar to previous observations in experimental animals. Three-compartment kinetic behaviour has been found in both rats and sheep. After an intravenous bolus dose to rats (30 µg kg−1 body weight) the half-lives were 00.25 h, 14 h, and 8.5 days [14]. In sheep, half-lives of 00.23 h, 7.9 h, and 2.5 days were observed [15]. However, the true terminal half-life was probably appreciably longer than 2.5 days, as the graphical estimation of the terminal half-life was clearly biased.
Time course of urinary excretion of vanadium
The cumulative urinary excretion of vanadium measured for 12 days is depicted in Figure 2. It is apparent that urinary excretion is the main elimination pathway for injected vanadium in humans. After 12 days about 24.9 µg, corresponding to 52% of the dose, had been recovered in urine. The time course of the cumulative amount of vanadium excreted in urine up to time t (Ut) could be described by the equation where λ1, λ2, λz are the rate constants describing the serum concentration-time profile (c.f. Table 1). U∞ = U1 + U2 + Uz is the estimated total amount of vanadium excreted in urine. The parameter values (mean and SEM) calculated by nonlinear regression were U1 = 3.9 ± 0.14 µg, U2 = 12.4 ± 0.31 µg, and U3 = 15.1 ± 0.56 µg. When interpreting the mass balance of administered vanadium one has to consider not only urinary excretion (52% of the dose after 12 days) but also the amount of vanadium remaining in the body. According to the definition of the volume of distribution, the amount of vanadium in the body after 12 days can be calculated from the product of the respective serum concentration (0.3 µg l−1) and Vz (55 l), which yields 16.5 µg. Together with the 24.9 µg recovered in urine after 12 days this amounts to some 41.4 µg. Thus about 13% of the total dose (47.6 µg) was unaccounted for, possibly reflecting the amount excreted in the faeces. It has been reported that the latter can account for about 10% of the excretion of vanadium administered i.v. in humans [34] and in experimental animals [35].
Figure 2.
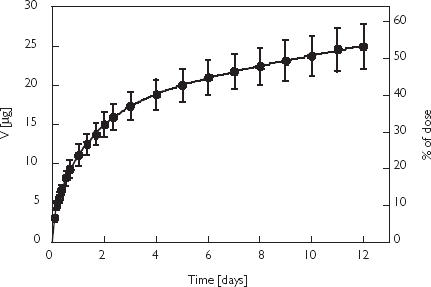
Cumulative urinary excretion of vanadium in five healthy volunteers after an intravenous infusion of 90 ml of an albumin solution containing 47.6 µg vanadium. Points are mean values ± SEM.
In the urine of a patient undergoing elective coronary revascularization who received an intravenous albumin solution (vanadium dose 47.4 µg or 0.6 µg kg−1 body weight), we observed a slightly higher renal excretion of vanadium (unpublished data). Fifty-eight per cent of the dose was recovered in urine after 5 days. We concluded that the increased excretion of vanadium during the first 5 days was caused by diuresis induced by the infusion solutions and diuretic drug both of which were administered postoperatively. In comparison with this patient, the cumulative urinary excretion of vanadium in the volunteers was between 28.9 and 54.3% during the same time period.
In a former study a higher urinary excretion of vanadium has been reported. In the two volunteers 81% of the intravenously administered dose (18 and 24 mg, respectively) was excreted in the urine by the 7th day after the last injection and 9% in the faeces over the same period. It was concluded that vanadium is excreted almost entirely by the kidney [34]. In conclusion, the present study has provided the first detailed information on the pharmacokinetics of vanadium in humans.
Acknowledgments
We thank the volunteers Mr Steffen K., Lars O., Dietmar H., Martin K., and Daniel S. for their participation and reliability. Dipl.-Phys. Matthias Mentler, Institute of Biophysics (Professor Dr F. Parak), Technical University of Munich, Germany, is acknowledged for measuring of the EPR spectra of vanadium in the albumin solutions. We thank Dr Michael Page, Ph.D. (Europäisches Patentamt, Munich, Germany) for linguistic comments. This study was not supported by any grant.
References
- 1.Nielsen FH, Uthus EO. The essentiality and metabolism of vanadium. In: Chasteen ND, editor. Vanadium in Biological Systems – Physiology and Biochemistry. Dordrecht: Kluwer Academic Publishers; 1990. pp. 51–62. [Google Scholar]
- 2.Nechay BR. Mechanisms of action of vanadium. Ann Rev Pharmacol Toxicol. 1984;24:501–524. doi: 10.1146/annurev.pa.24.040184.002441. [DOI] [PubMed] [Google Scholar]
- 3.Schaller KH, Triebig G. Vanadium. In: Alessio L, Berlin A, Boni M, Roi R, editors. Biological Indicators for the Assessment of Human Exposure to Industrial Chemicals. Ispra: Commission of the European Communities; 1987. pp. 78–93. [Google Scholar]
- 4.Willsky GR. Vanadium in the biosphere. In: Chasteen ND, editor. Vanadium in Biological Systems – Physiology and Biochemistry. Dordrecht: Kluwer Academic Publishers; 1990. pp. 1–24. [Google Scholar]
- 5.Sigel H, Sigel A. Metal ions in biological systems. Vol. 31. New York: Marcel Dekker; 1995. Vanadium and its role in life; pp. 1–779. [Google Scholar]
- 6.Nriagu JO. Advances in Environmental Science and Technology. New York: John Wiley & Sons; 1998. Vanadium in the environment. Part 2: Health effects; pp. 1–403. [Google Scholar]
- 7.Tracey AS, Crans DC. ACS Symposium Series 711. Washington: American Chemical Society; 1998. Vanadium Compounds: Chemistry, Biochemistry, and Therapeutic Applications; pp. 1–403. [Google Scholar]
- 8.Cohen N, Halberstam M, Shlimovich P, et al. Oral vanadyl sulfate improves hepatic and peripheral insulin sensitivity in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1995;95:2501–2509. doi: 10.1172/JCI117951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldfine AB, Simonson DC, Folli F, Patti ME, Kahn CR. Metabolic effects of sodium metavanadate in humans with insulin-dependent diabetes mellitus:in vivo and in vitro studies. J Clin Endocrinol Metab. 1995;80:3311–3320. doi: 10.1210/jcem.80.11.7593444. [DOI] [PubMed] [Google Scholar]
- 10.Boden G, Chen X, Ruiz J, van Rossum GD, Turco S. Effects of vanadyl sulfate on carbohydrate and lipid metabolism in patients with non-insulin-dependent diabetes mellitus. Metabolism. 1996;45:1130–1135. doi: 10.1016/s0026-0495(96)90013-x. [DOI] [PubMed] [Google Scholar]
- 11.Thompson KH, Orvig C. Design of vanadium compounds as insulin enhancing agents. J Chem Soc-Dalton Trans. 2000. pp. 2885–2892.
- 12.Heinemann G, Vogt W. Quantification of vanadium in serum by electrothermal atomic absorption spectrometry. Clin Chem. 1996;42:1275–1282. [PubMed] [Google Scholar]
- 13.Oberg SG, Parker RD, Sharma RP. Distribution and elimination of an intratracheally administered vanadium compound in the rat. Toxicology. 1978;11:315–323. doi: 10.1016/s0300-483x(78)91889-9. [DOI] [PubMed] [Google Scholar]
- 14.Sabbioni E, Marafante E. Metabolic patterns of vanadium in the rat. Bioinorg Chem. 1978;9:389–407. doi: 10.1016/0006-3061(78)80004-0. [DOI] [PubMed] [Google Scholar]
- 15.Hansard SL, Ammerman CB, Henry PR, Patterson BW. Vanadium metabolism in sheep. III. Influence of dietary vanadium on kinetics of 48V administered orally or intravenously and comparison of compartmental and graphical models. J Animal Sci. 1986;62:804–812. doi: 10.2527/jas1986.623804x. [DOI] [PubMed] [Google Scholar]
- 16.Setyawati IA, Thompson KH, Yuen VG, et al. Kinetic analysis and comparison of uptake, distribution, and excretion of V-48-labeled compounds in rats. J Appl Physiol. 1998;84:569–575. doi: 10.1152/jappl.1998.84.2.569. [DOI] [PubMed] [Google Scholar]
- 17.Sakurai H, Sano H, Takino T, Yasui H. A new type of orally active insulin-mimetic vanadyl complex: bis (1-oxy-2-pyridinethiolato) oxovanadium (IV) with VO (S2O2) coordination mode. Chem Lett. 1999;9:913–914. [Google Scholar]
- 18.Sakurai H, Sano H, Takino T, Yasui H. An orally active antidiabetic vanadyl complex, bis (1-oxy-2- pyridinethiolato) oxovanadium (IV), with VO (S2O2) coordination mode; in vitro and in vivo evaluations in rats. J Inorg Biochem. 2000;80:99–105. doi: 10.1016/s0162-0134(00)00045-3. [DOI] [PubMed] [Google Scholar]
- 19.Yasui H, Takechi K, Sakurai H. Metallokinetic analysis of disposition of vanadyl complexes as insulin-mimetics in rats using BCM-ESR method. J Inorg Biochem. 2000;78:185–196. doi: 10.1016/s0162-0134(00)00002-7. [DOI] [PubMed] [Google Scholar]
- 20.Derendorf H, Garrett ER. Pharmakokinetik. Stuttgart: Wiss-Verlagsges; 1987. [Google Scholar]
- 21.Ritschel WA. Handbook of Basic Pharmacokinetics. Hamilton, IL, USA: Drug Intelligence Publications; 1977. pp. 1–370. [Google Scholar]
- 22.Allen L, Kimura K, MacKichan JJ, Ritschel WA. Manual of symbols, equations and definitions in pharmakokinetics. J Clin Pharmacol. 1982;22:1S–23S. [PubMed] [Google Scholar]
- 23.Hopkins LL, Jr, Tilton BE. Metabolism of trace amounts of vanadium 48 in rat organs and liver subcellular particles. Am J Physiol. 1966;211:169–172. doi: 10.1152/ajplegacy.1966.211.1.169. [DOI] [PubMed] [Google Scholar]
- 24.Conklin AW, Skinner SC, Felten TL, Sanders CL. Clearance and distribution of intratracheally instilled vanadium compounds in the rat. Toxicol Lett. 1982;11:199–203. doi: 10.1016/0378-4274(82)90128-x. [DOI] [PubMed] [Google Scholar]
- 25.Kucera J, Simkova M, Lener J, et al. Vanadium determination in rat tissues and biological reference materials by neutron activation analysis. J Radioanal Nucl Chem. 1990;141:49–59. [Google Scholar]
- 26.Heinemann G, Fichtl B, Mentler M, Vogt W. Binding of vanadate to human albumin in infusion solutions, to proteins in human fresh frozen plasma, and to transferrin. J Inorg Biochem. 2002;90:38–42. doi: 10.1016/s0162-0134(02)00399-9. [DOI] [PubMed] [Google Scholar]
- 27.Chasteen ND, Grady JK, Holloway CE. Characterization of the binding, kinetics, and redox stability of vanadium (IV) and vanadium (V) protein complexes in serum. Inorg Chem. 1986;25:2754–2760. [Google Scholar]
- 28.Purcell M, Neault JF, Malonga H, Arakawa H, Tajmir-Riahi HA. Interaction of human serum albumin with oxovanadium ions studied by FT-IR spectroscopy and gel and capillary electrophoresis. Can J Chem. 2001;79:1415–1421. [Google Scholar]
- 29.Nielsen FH. Vanadium in mammalian physiology and nutrition. In: Sigel H, Sigel A, editors. Vanadium and its role in life. New York: Marcel Dekker; 1995. pp. 543–573. [Google Scholar]
- 30.Stankiewicz PJ, Tracey AS. Stimulation of enzyme activity by oxovanadium complexes. In: Sigel H, Sigel A, editors. Vanadium and its role in life. New York: Marcel Dekker; 1995. pp. 249–285. [PubMed] [Google Scholar]
- 31.Cannon JC, Chasteen ND. Nonequivalence of the metal binding sites in vanadyl-labeled human serum transferrin. Biochemistry. 1975;14:4573–4577. doi: 10.1021/bi00692a003. [DOI] [PubMed] [Google Scholar]
- 32.Sabbioni E, Marafante E, Pietra R, et al. The association of vanadium with the iron transport system in human blood as determined by gel filtration and neutron activation analysis. Proceedings of the International Symposium on Nuclear Activation Techniques in the Life Sciences, Wien. 1978. pp. 179–192.
- 33.Harris WR, Carrano CJ. Binding of vanadate to human serum transferrin. J Inorg Biochem. 1984;22:201–218. doi: 10.1016/0162-0134(84)80029-x. [DOI] [PubMed] [Google Scholar]
- 34.Kent NL, McKane RA. The absorption and excretion of ‘minor’ elements by man. 1. Silver, gold, lithium, boron and vanadium. Biochem J. 1941;35:837–844. doi: 10.1042/bj0350837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willsky GR, Goldfine AB, Kostyniak PJ. Pharmacology and toxicology of oxovanadium species: Oxovanadium pharmacology. In: Tracey AS, Crans DC, editors. Vanadium Compounds: Chemistry, Biochemistry, and Therapeutic Applications. Washington: American Chemical Society; 1998. pp. 278–296. [Google Scholar]


