Abstract
Aims
To determine CYP2C19 and CYP2D6 activity in patients with multiple sclerosis (MS) before and during interferon (IFN)-β treatment.
Methods
CYP2C19 and CYP2D6 activities were assessed using the probe drugs mephenytoin and debrisoquine, respectively. Urinary mephenytoin (S/R) and debrisoquine (debrisoquine/hydroxy-debrisoquine) metabolic ratios (MR) were determined in 10 otherwise healthy Caucasian multiple sclerosis (MS) patients in the initial stage of the disease, prior to and 1 month after commencing treatment with IFN-β (Avonex, Rebif or Betaferon). In addition, CYP2C19*2, CYP2C19*3, CYP2D6*3, CYP2D6*4, and CYP2D6*5 genotyping was performed.
Results
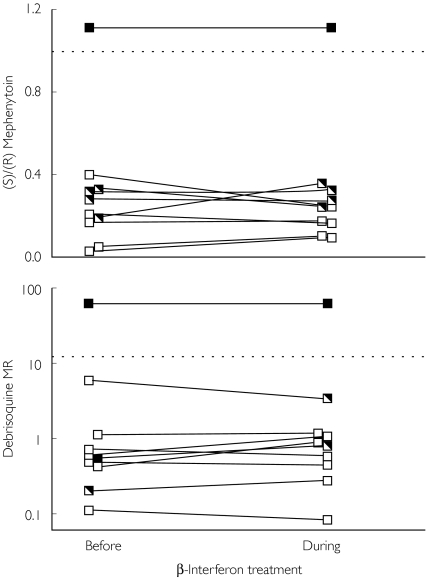
There was no significant difference in the (S)/(R) mephenytoin ratio (mean difference 0.04; 95% CI −0.03, 0.11) or the debrisoquine MR (mean difference 0.29; 95% CI −0.44, 1.02) before and during regular IFN-β treatment in extensive metabolizers (EM) (P = 0.5 and P = 0.4 for the respective probe drugs; n = 9 subjects). There were also no differences between the different IFN-β treatments (P = 0.6 for the (S)/(R) mephenytoin ratio and P = 0.7 for the debrisoquine MR; anova; n = 10).
Conclusions
IFN-β treatment did not affect the activity of CYP2C19 or CYP2D6. The results suggest that it is safe to administer CYP2C19 or CYP2D6 substrates, without dose adjustment, to patients treated with IFN-β.
Keywords: CYP2C19, CYP2D6, cytochrome P-450, interferon-β, multiple sclerosis
Introduction
The urinary S/R mephenytoin and debrisoquine metabolic ratios (MR), are used to characterize in vivo activity of the polymorphic enzymes CYP2C19 [1] and CYP2D6 [2], respectively. Subjects with markedly impaired activity of these enzymes are termed poor metabolizers (PM). The prevalence of CYP2C19 and CYP2D6 PM phenotypes in Caucasian subjects is 3% and 7%, respectively, but differs between ethnic groups. Apart from genetic factors, the activity of these enzymes is influenced by environmental factors (e.g. drug–drug interactions) and disease states, for example infection and inflammation [3]. Multiple sclerosis (MS) affects approximately 1 in 1000 people. The inflammatory and demyelinating lesions of MS cause relapsing episodes of neurological dysfunction. Interferon-β (IFN-β) has immunomodulatory properties that have been shown to alter the course of MS by reducing clinical relapse rate and delaying disability progression [4].
Many commonly used drugs, including antidepressants, benzodiazepines, β-adrenoceptor blockers, and proton pump inhibitors, are metabolized by CYP2C19 or CYP2D6. Reports indicate that IFN could cause a clinically significant interaction when given with drugs that are CYP substrates. IFN-α administration impairs the clearance of antipyrine [5] and that of erythromycin [6] in hepatitis B patients. In another study patients receiving an optimal maintenance dose of warfarin needed a dose reduction when IFN-α-2b or IFN-β was given [7] and potentiation of anticoagulation was described after giving IFN-α-2b to a woman who had been medicated with acenocoumarol for 20 years [8]. In another study the elimination half-life of theophylline increased 1.5- to 6-fold when given with IFN [9].
The aim of the present study was to establish whether the forms of interferon affect the activity of CYP2C19 or CYP2D6. This is an important issue, since the use of IFN therapy in MS and other diseases, such as leukaemia, myeloma and solid tumours, herpes simplex infections, and hepatitis B and C, is expected to increase in the future, and patients are often treated with one or more CYP2C19 and CYP2D6 substrates.
Methods
Patients
Ten Caucasian subjects with MS (eight women and two men aged 27–50 years) were studied. All patients were newly diagnosed with relapsing-remitting MS and were in the first stage of the disease. None had moderate or severe disability. All patients were clinically stable throughout the study. The majority had normal liver function tests (alanine aminotransferase (ALT) normal limits <0.6 mkat l−1 for females; < 0.8 mkat l−1 for males), except for one female patient who had a modestly raised ALT value after 4 weeks (0.7), and two other female patients who had increased values initially (0.9 and 1.1, respectively). In these cases, values became normal after 4 weeks. The probe drugs; 100 mg racemic mephenytoin (Mesantoin®; Sandoz AG, Basel, Switzerland) and 10 mg debrisoquine (Declinax®; Hoffmann-La Roche AG, Basel, Switzerland) were taken before bedtime and all urine was collected for the subsequent 8 h. The following day the patients brought their urine samples to the Department of Neurology, where a 10 ml blood sample for genotyping was drawn. At that visit the patients were taught how to self administer IFN intramuscularly (i.m.) or subcutaneously (s.c.). After 1 month of regular IFN-β administration, the phenotyping procedure was repeated.
Patients received either IFN-β-1a as Avonex 30 µg weekly i.m. (Biogen, Nanterre, France) or as Rebif 22 or 44 µg thrice weekly s.c. (Serono International AG, Geneva, Switzerland), or IFN-β-1b as Betaferon 250 µg every other day s.c. (Schering AG, Berlin, Germany). The IFN-β preparation was chosen by the treating neurologist after discussion with the patient. Additional treatment, including two subjects on hormone replacement therapy, one taking the oral contraceptive, one taking propranolol, one taking atenolol, two taking of levaxin and two taking vitamins, remained the same throughout the study.
Alcohol and over-the-counter drugs were not allowed during study days. The patients consented in writing to participate after a full explanation of the procedures. The study protocol was approved by the Human Ethics Committee at Karolinska Institutet, Huddinge University Hospital in Stockholm, Sweden.
Genotyping and drug determination
Genotypes were determined by PCR analysis of the CYP2C19*2 and*3 [10] and CYP2D6*3, *4 and *5 alleles [11]. (S) and (R) mephenytoin were measured by gas chromatography [12]. Debrisoquine and hydroxydebrisoquine were quantified by HPLC [13]. Samples were assayed in singlicate. The interassay coefficient of variation for (S) and (R) mephenytoin was less than 5%, for debrisoquine 5.1% (3 µm), 5.7% (30 µm) and 6.4% (80 µm), and for hydroxydebrisoquine 8.5% (3 µm), 5.8% (30 µm), and 12.1% (80 µm). The antimodes for assigning phenotypes were unity for the (S)/(R) mephenytoin ratio and 12 for the debrisoquine MR. In white subjects, genotypic extensive metabolizers (EM) carry either no or one mutation(s), whereas poor metabolizers (PM) typically have two mutations of the CYP2D6 or CYP2C19 gene.
Statistics
The power calculation was based on a study showing a 30% decrease of antipyrine oral clearance after chronic treatment with IFN-α [14]. We estimated that nine subjects are needed to have 80% power to detect a 30% increase in the metabolic ratio.
A paired t-test was used for comparison of the (S)/(R) mephenytoin ratio and the debrisoquine MR before and during IFN-β treatment. anova was used to compare the effect of the different IFN-β preparations. P < 0.05 was considered a significant difference.
Results
There was no significant difference in the (S)/(R) mephenytoin ratio (P = 0.5, n = 9 subjects) (mean difference 0.04; 95% CI −0.03, 0.11) (Figure 1) or the debrisoquine MR (P = 0.4, n = 9 subjects) (mean difference 0.29; 95% CI −0.44, 1.02) before and during regular IFN-β treatment in genotypic EM. In the single PM, no difference in either ratio was observed.
Figure 1.
(S)/(R) mephenytoin ratio (upper panel) and debrisoquine metabolic ratio (MR) (lower panel) before and during administration of interferon (IFN)-β in patients with MS with two, one, or zero wild-type (wt) or mutated (mut) alleles, respectively. The different CYP2C19 and CYP2D6 genotypes are indicated as filled, semi or unfilled squares and refer to the CYP2C19*2 (upper panel) and the CYP2D6*3 or CYP2D6*4 (lower panel) mutations, respectively. The dotted lines indicate the antimodes of the two ratios. There was no significant difference in the (S)/(R) mephenytoin ratio (mean difference 0.04; 95% CI −0.03, 0.11) or the debrisoquine MR (0.29; 95% CI −0.44, 1.02) before and during regular IFN-β treatment in EM (P = 0.5 and P = 0.4 for the respective probe drugs; n = 9 subjects). There was a complete concordance between geno- and phenotype for both enzymes. Mut/mut (▪); wt/mut ( ); and wt/wt (□).
); and wt/wt (□).
There was no difference in CYP2C19 or CYP2D6 activity between patients who were treated with the different forms of IFN-β (P = 0.6 for the (S)/(R) mephenytoin and P = 0.7 for the debrisoquine MR, n = 10 subjects).
There was complete concordance between geno- and phenotypes for both CYP2C19 and CYP2D6.
Discussion
In the present work we found that IFN-β treatment has no effect on CYP2C19 and CYP2D6 activity. In a previous study of seven cancer patients, IFN-α did not change the metabolism of hexobarbitone, but it did alter that of theophylline and antipyrine. Thus IFN-α seems to inhibit the hepatic metabolism of some but not all drugs [14].
Several factors may have contributed to the lack of detectable effects of IFN-β on CYP2C19 or CYP2D6 activity. However it is clear from Figure 1 that the two indices of CYP activity are very stable within patients, i.e. before and during IFN-β treatment. This is in agreement with a study showing that both the S/R mephenytoin ratio and the debrisoquine MR do not change significantly over time within individuals [16]. Thus, a relatively small effect of IFN-β treatment would have been detected in our study. It seems likely that different CYP enzymes are affected differently by IFN, and it is possible that enzymes other than CYP2D6 and CYP2C19 are affected in MS patients receiving IFN-β. It is also possible that CYP2C19 and CYP2D6 inhibition by IFN does actually occur, but through a time course not detectable by the present study design. Furthermore, patients with MS may be more susceptible to inhibition at later stages of their disease.
Since enzymatic activity of CYP2C19 and CYP2D6 in PM is markedly reduced, no difference in enzyme capacity during treatment with IFN was expected or noted in this study.
In conclusion, the major finding of this study was that regular administration of IFN-β did not cause any change in the (S)/(R) mephenytoin ratio or the debrisoquine MR in MS patients. Thus the data suggest that dose adjustments of drugs metabolized by CYP2C19 or CYP2D6 are not indicated. However, further studies are warranted to investigate the function of these and other drug metabolizing enzymes in MS patients in later stages of the disease, and after long-term regular administration with IFN-β.
Acknowledgments
We acknowledge the participation of the patients in the study and the co-operative work of RN Katarina Andersson. This work was supported by Otto Swärd's Foundation and the Swedish Medical Research Council (3902). K.H. is supported by a postdoctoral fellowship from the Wenner-Gren Foundation which is gratefully acknowledged.
References
- 1.Kupfer A, Preisig R. Pharmacogenetics of mephenytoin: a new drug hydroxylation polymorphism in man. Eur J Clin Pharmacol. 1984;26:753–759. doi: 10.1007/BF00541938. [DOI] [PubMed] [Google Scholar]
- 2.Mahgoub A, Idle JR, Dring LG, Lancaster R, Smith RL. Polymorphic hydroxylation of debrisoquine in man. Lancet. 1977;ii:584–586. doi: 10.1016/s0140-6736(77)91430-1. [DOI] [PubMed] [Google Scholar]
- 3.Morgan ET. Regulation of cytochromes P450 during inflammation and infection. Drug Metab Rev. 1997;29:1129–1188. doi: 10.3109/03602539709002246. [DOI] [PubMed] [Google Scholar]
- 4.Noseworthy JH. Progress in determining the causes and treatment of multiple sclerosis. Nature. 1999;399:A40–A47. doi: 10.1038/399a040. [DOI] [PubMed] [Google Scholar]
- 5.Williams SJ, Farrell GC. Inhibition of antipyrine metabolism by interferon. Br J Clin Pharmacol. 1986;22:610–612. doi: 10.1111/j.1365-2125.1986.tb02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig PI, Tapner M, Farrell GC. Interferon suppresses erythromycin metabolism in rats and human subjects. Hepatology. 1993;17:230–235. [PubMed] [Google Scholar]
- 7.Adachi Y, Yokoyama Y, Nanno T, Yamamoto T. Potentiation of warfarin by interferon. Br Med J. 1995;311:292. doi: 10.1136/bmj.311.7000.292a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serratrice J, Durand JM, Morange S. Interferon–alpha 2b interaction with acenocoumarol. Am J Hematol. 1998;57:89. doi: 10.1002/(sici)1096-8652(199801)57:1<89::aid-ajh18>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Williams SJ, Baird-Lambert JA, Farrell GC. Inhibition of theophylline metabolism by interferon. Lancet. 1987;ii:939–941. doi: 10.1016/s0140-6736(87)91422-x. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein JA, Ishizaki T, Chiba K, et al. Frequencies of the defective CYP2C19 alleles responsible for the mephenytoin poor metabolizer phenotype in various Oriental, Caucasian, Saudi Arabian and American black populations. Pharmacogenetics. 1997;7:59–64. doi: 10.1097/00008571-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Dahl ML, Johansson I, Palmertz MP, Ingelman-Sundberg M, Sjoqvist F. Analysis of the CYP2D6 gene in relation to debrisoquine and desipramine hydroxylation in a Swedish population. Clin Pharmacol Ther. 1992;51:12–17. doi: 10.1038/clpt.1992.2. [DOI] [PubMed] [Google Scholar]
- 12.Wedlund PJ, Sweetman BJ, McAllister CB, Branch RA, Wilkinson GR. Direct enantiomeric resolution of mephenytoin and its N-demethylated metabolite in plasma and blood using chiral capillary gas chromatography. J Chromatogr. 1984;307:121–127. doi: 10.1016/s0378-4347(00)84078-5. [DOI] [PubMed] [Google Scholar]
- 13.Svensson JO, Bertilsson L. Rapid high-performance liquid chromatographic method for determination of debrisoquine and 4-hydroxy-debrisoquine in urine for CYP2D6 phenotyping. Pharmacogenetics. 1999;9:529–531. [PubMed] [Google Scholar]
- 14.Israel BC, Blouin RA, McIntyre W, Shedlofsky SI. Effects of interferon-alpha monotherapy on hepatic drug metabolism in cancer patients. Br J Clin Pharmacol. 1993;36:229–235. doi: 10.1111/j.1365-2125.1993.tb04222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anonymous. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology. 1993;43:655–661. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- 16.Brynne N, Bottiger Y, Hallen B, Bertilsson L. Tolterodine does not affect the human in vivo metabolism of the probe drugs caffeine, debrisoquine and omeprazole. Br J Clin Pharmacol. 1999;47:145–150. doi: 10.1046/j.1365-2125.1999.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkinson A, Lasker J, Kramer MJ, et al. Effects of three recombinant human leukocyte interferons on drug metabolism in mice. Drug Metab Dispos. 1982;10:579–585. [PubMed] [Google Scholar]