Abstract
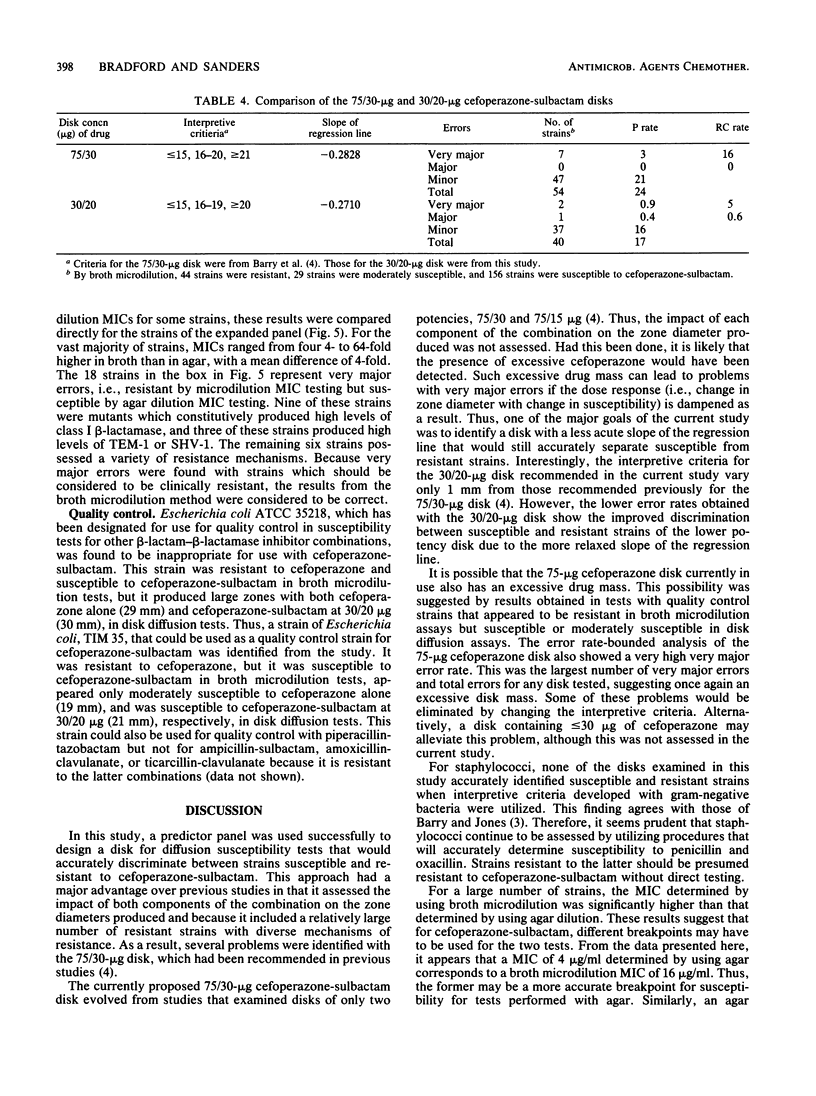
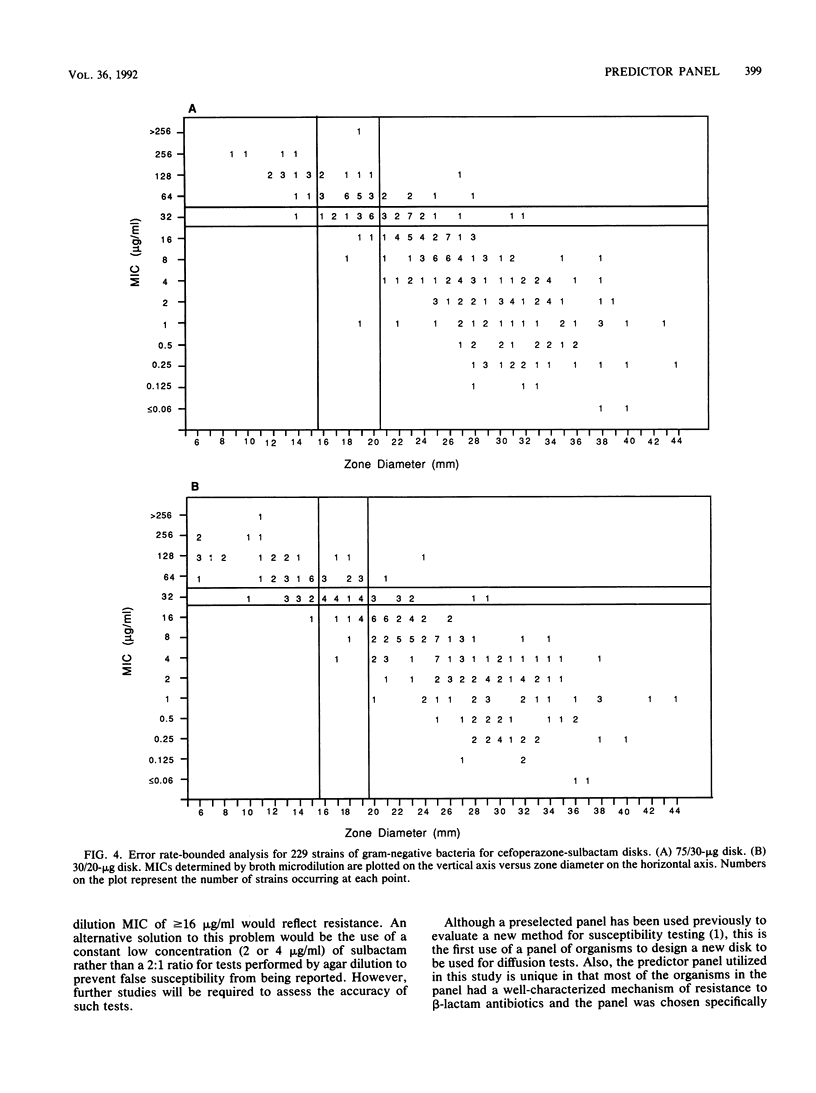
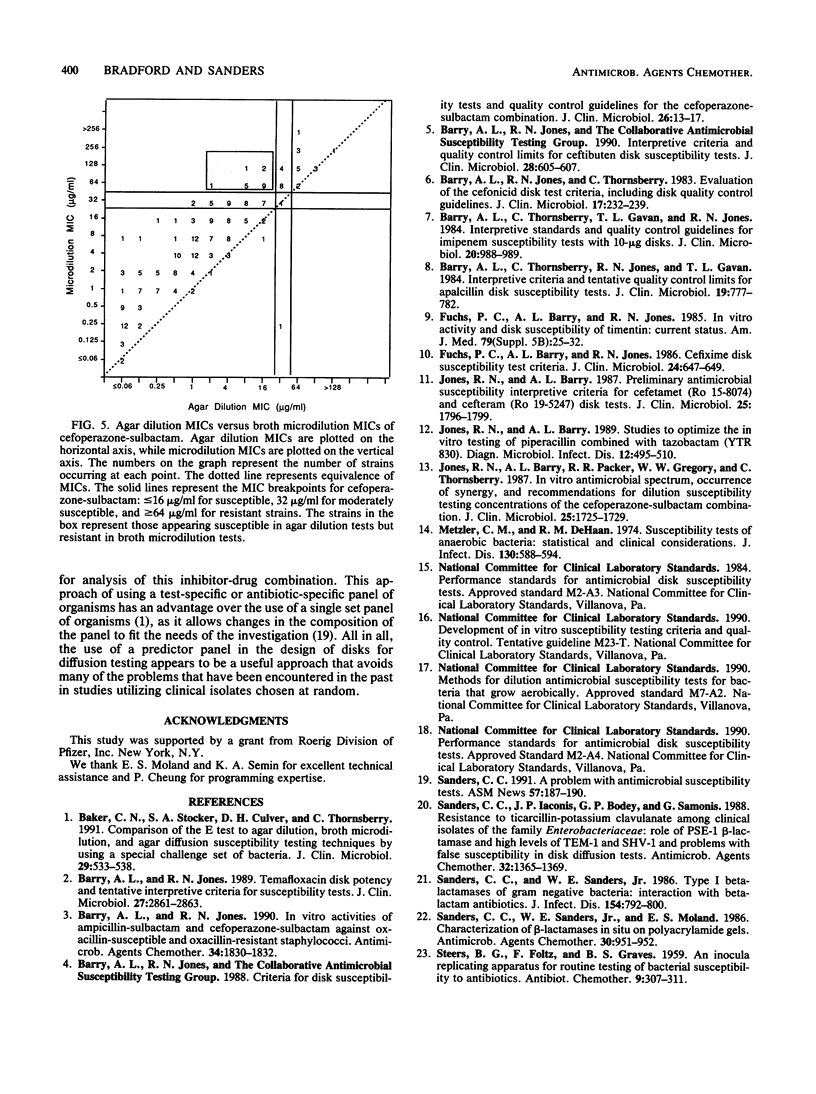
The proper disk mass for diffusion susceptibility tests with cefoperazone-sulbactam was determined by using a predictor panel of clinical isolates that included staphylococci and gram-negative bacteria intrinsically susceptible, intrinsically resistant, and of various susceptibilities because of the production of different types and amounts of beta-lactamase. A primary panel of 24 isolates was used to screen various disk masses of cefoperazone and sulbactam in disk diffusion susceptibility tests. Regression analyses were performed for each combination by comparing MICs to zone diameters. Analysis of each component demonstrated that decreasing the disk mass of cefoperazone shifted the regression line to the left while decreasing the disk mass of sulbactam diminished the slope of the line. Ten candidate disks that adequately separated susceptible and resistant strains among the primary panel were identified, and these 10 disks, along with the previously proposed 75/30-micrograms disk, were then tested against an expanded panel of 265 isolates. Results indicated that a 30/20-micrograms cefoperazone-sulbactam disk provided the best separation between susceptible and resistant strains when interpretive criteria of less than or equal to 15 mm for resistance, 16 to 19 mm for moderate susceptibility, and greater than or equal to 20 mm for susceptibility were used. They also identified discrepancies between agar and broth microdilution MICs of sufficient size to warrant separate interpretive criteria for the two methods. Overall, the use of a predictor panel to develop interpretive criteria for susceptibility tests appeared to be a very useful approach, especially when antibiotics designed to be used against drug-resistant organisms are involved.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. N., Stocker S. A., Culver D. H., Thornsberry C. Comparison of the E Test to agar dilution, broth microdilution, and agar diffusion susceptibility testing techniques by using a special challenge set of bacteria. J Clin Microbiol. 1991 Mar;29(3):533–538. doi: 10.1128/jcm.29.3.533-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N. Criteria for disk susceptibility tests and quality control guidelines for the cefoperazone-sulbactam combination. J Clin Microbiol. 1988 Jan;26(1):13–17. doi: 10.1128/jcm.26.1.13-17.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N. In vitro activities of ampicillin-sulbactam and cefoperazone-sulbactam against oxacillin-susceptible and oxacillin-resistant staphylococci. Antimicrob Agents Chemother. 1990 Sep;34(9):1830–1832. doi: 10.1128/aac.34.9.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N. Interpretive criteria and quality control limits for ceftibuten disk susceptibility tests. Collaborative Antimicrobial Susceptibility Testing Group. J Clin Microbiol. 1990 Mar;28(3):605–607. doi: 10.1128/jcm.28.3.605-607.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N. Temafloxacin disk potency and tentative interpretive criteria for susceptibility tests. J Clin Microbiol. 1989 Dec;27(12):2861–2863. doi: 10.1128/jcm.27.12.2861-2863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N., Thornsberry C. Evaluation of the cefonicid disk test criteria, including disk quality control guidelines. J Clin Microbiol. 1983 Feb;17(2):232–239. doi: 10.1128/jcm.17.2.232-239.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Gavan T. L., Jones R. N. Interpretive standards and quality control guidelines for imipenem susceptibility tests with 10-micrograms disks. J Clin Microbiol. 1984 Nov;20(5):988–989. doi: 10.1128/jcm.20.5.988-989.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Jones R. N., Gavan T. L. Interpretive criteria and tentative quality control limits for apalcillin disk susceptibility tests. J Clin Microbiol. 1984 Jun;19(6):777–782. doi: 10.1128/jcm.19.6.777-782.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. C., Barry A. L., Jones R. N. Cefixime disk susceptibility test criteria. J Clin Microbiol. 1986 Oct;24(4):647–649. doi: 10.1128/jcm.24.4.647-649.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. C., Barry A. L., Jones R. N. In vitro activity and disk susceptibility of Timentin: current status. Am J Med. 1985 Nov 29;79(5B):25–32. doi: 10.1016/0002-9343(85)90125-1. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Packer R. R., Gregory W. W., Thornsberry C. In vitro antimicrobial spectrum, occurrence of synergy, and recommendations for dilution susceptibility testing concentrations of the cefoperazone-sulbactam combination. J Clin Microbiol. 1987 Sep;25(9):1725–1729. doi: 10.1128/jcm.25.9.1725-1729.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. Preliminary antimicrobial susceptibility interpretive criteria for cefetamet (Ro 15-8074) and cefteram (Ro 19-5247) disk tests. J Clin Microbiol. 1987 Sep;25(9):1796–1799. doi: 10.1128/jcm.25.9.1796-1799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. Studies to optimize the in vitro testing of piperacillin combined with tazobactam (YTR 830). Diagn Microbiol Infect Dis. 1989 Nov-Dec;12(6):495–510. doi: 10.1016/0732-8893(89)90084-9. [DOI] [PubMed] [Google Scholar]
- Metzler C. M., DeHaan R. M. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J Infect Dis. 1974 Dec;130(6):588–594. doi: 10.1093/infdis/130.6.588. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Iaconis J. P., Bodey G. P., Samonis G. Resistance to ticarcillin-potassium clavulanate among clinical isolates of the family Enterobacteriaceae: role of PSE-1 beta-lactamase and high levels of TEM-1 and SHV-1 and problems with false susceptibility in disk diffusion tests. Antimicrob Agents Chemother. 1988 Sep;32(9):1365–1369. doi: 10.1128/aac.32.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Moland E. S. Characterization of beta-lactamases in situ on polyacrylamide gels. Antimicrob Agents Chemother. 1986 Dec;30(6):951–952. doi: 10.1128/aac.30.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Type I beta-lactamases of gram-negative bacteria: interactions with beta-lactam antibiotics. J Infect Dis. 1986 Nov;154(5):792–800. doi: 10.1093/infdis/154.5.792. [DOI] [PubMed] [Google Scholar]