Abstract
The β-1 adrenoceptor is an archetypal G-coupled protein receptor that controls sympathetic responses in the heart, kidney and adipocytes. It has been widely exploited as a drug target with the development of antagonists to treat cardiovascular diseases such as hypertension, angina and heart failure. Signalling through the receptor is modulated by desensitization and β1- adrenoceptor down-regulation. It is also affected by in vitro substitution of specific amino acid residues within the β-1 adrenoceptor. Amino acid substitutions also occur naturally due to polymorphic variation within the human β-1 adrenoceptor gene itself. Since these variants are common (typically being present in >5% of the population), the pharmacogenetic implications are enormous. A number of these variants have been identified, although two have been the particular focus of recent publications: a serine to glycine substitution at position 49 (49S > G) and an arginine to glycine at position 389 (389R > G). The data on the in vitro behaviour of these two receptor variants is reviewed here, along with the evidence that they may affect both the risk of cardiovascular disease and the therapeutic response to β-1 adrenoceptor antagonists.
Introduction
G-protein coupled receptors (GPCRs) with ∼750 members represent one of the largest superfamilies of genes in the genome. They allow integrated and dynamic signalling to maintain homeostasis in response to physiological and pathological events. It is now clear that single nucleotide polymorphisms (SNPs) or variations are frequent within the genes for these receptors. Coding SNPs (cSNPs) that affect the amino acids encoded into the GPCR receptor protein are of particular interest, since they can potentially affect the responses to drugs or endogenous agonists and therefore alter the pathophysiology of specific disease states. This is especially important for the β1-adrenoceptor (AR), which mediates many of the effects of endogenous catecholamines that regulate key physiological events involving the heart, kidney and adipocytes: namely heart rate and contractility, renin release and lipolysis [1–3]. The β1-AR gene is reported to contain up to 12 cSNPs within its single coding exon [4]. However, recent interest has focused on the two common polymorphisms for this receptor, 389Arg > Gly (389 R > G) and 49Gly > Ser (49 G > S). This review discusses the work establishing a functional role for these β1-AR polymorphisms and the part they may play in the pathophysiology of complex disease traits involving the β1-AR. This pharmacogenetic view of the β1-AR could improve therapeutic targeting for these diseases and patient risk stratification.
The β1-adrenoceptor (β1-AR)
The β1-AR is a typical example of the TM-7 family of adrenergic receptors that were originally classified on the basis of tissue responses to archetypal sympathomimetic agonists [5, 6]. It is activated by enveloping the agonist into a pocket allowing conformational changes to the integral part of the receptor, the G-protein binding domain. The resulting complex regulates effector molecules in close proximity to the binding domain leading to tissue responses [7, 8]. Activation can lead to beneficial physiological responses, but sustained stimulation plays a key role in the development and progression of cardiovascular disease. To some extent the deleterious effects of chronic adrenergic signalling are modulated by desensitization and down regulation of the β1-AR [9]. However, pharmacological blockade of the β1-AR has become routine in the treatment of ischaemic heart disease, hypertension and heart failure reflecting the pathophysiological importance attached to this receptor.
The β1-AR was first cloned from a human placenta cDNA library and localized to chromosome 10 (10q24-q26) using somatic cell hybrid analysis and in situ hybridization [10, 11]. The subsequent construction of chimeric β1/β2 receptors and the mutation of individual residues or distinct regions of the β1-AR have led to a detailed understanding of its various functional domains. For example, transmembrane spanning region IV appears to be the most important determinant of receptor subtype specificity [12]. The third intracellular loop, or more specifically a proline rich region of 24 amino acids within it, is responsible for the less efficient coupling of the β1-AR to Gs (compared with the β2-AR) and also affects agonist promoted sequestration [13]. Various levels of constitutive activity are also produced by the replacement of the C-terminal residue of the third intracellular loop (L322) with amino acids differing in their physico-chemical properties [14]. The C-terminal domain itself determines subtype specific (β1vs.β2) desensitization patterns when expressed in Chinese hamster and murine fibroblasts [15]. The N-terminal is also functionally important in addition to its phosphorylation and association with cytoplasmic proteins. Hence, mutation of the asparagine residue at position 15 (15 N > A) affects β1-AR surface expression and dimerization compared with wild type β1-AR confirming it as a key N-glycosylation site [16].
Variability of response to β1-AR agonists and antagonists
The responsiveness to β1-AR antagonist treatment, endogenous catecholamines and susceptibility to cardiac disease show considerable variability between individuals [17–20]. This was initially assumed to be due to disease status or post-translational modification of receptor function [21], coupled with changes in the β1-AR signal transduction during progression of diseases such as heart failure [22]. However, it is now apparent that molecular genetics provides at least a partial understanding of interindividual differences, which remain even allowing for obvious confounders such as age, sex, pharmacokinetics, and aetiology/severity of cardiac disease. This situation parallels the impact that pharmacogenetics has had on the closely related β2-AR [23].
Polymorphisms within the β1-AR gene (ADRB1)
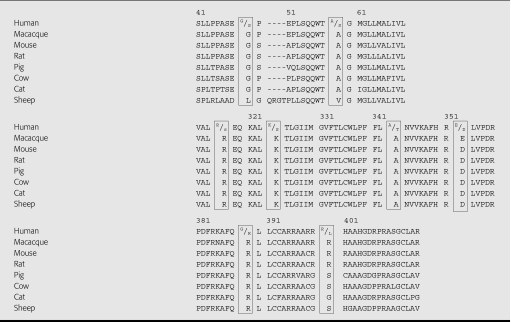
A polymorphism was actually detected in the β1-AR gene shortly after it was first cloned using the same 2.4kb clone to Southern blot BglI digests of genomic DNA from healthy subjects [24]. However it was another decade before the human β1-AR gene was subjected to systematic mutation scanning and sequencing [25–28]. Currently, the NCBI SNP database reports 12 cSNPs of which eight are nonsynonymous and result in an amino acid substitution (see Table 1). However, it must be emphasized that most of these SNPs have not been formally verified and exact allele frequencies are unavailable. Nevertheless, it is interesting that all of the nonsynonymous SNPs are located within regions that are highly conserved across species (Table 2). A further 16 SNPs have been reported by Podlowski from mutation scanning of patients with idiopathic dilated cardiomyopathy (IDCM) and normal volunteers without evidence of heart disease [29]. These included one located in the N-terminal: G > T exchange at position 175 (59Ala > Ser). The remainder were identified within the C terminus: 1195C > T (399Arg > Cys), 1205 A > G at 1205 (402His > Arg), 1210 A > G (404Thr > Ala) and 1252C > G (418Pro > Ala). However, they are all rare (frequencies of 1–2%) and have not been verified in other studies. Finally, in the 5′ flanking region, a single individual displayed a homozygous C-T exchange at position −38. This same group has reported three further variants in the 5′ flanking region of ADRB1 in patients with IDCM and coronary heart disease (−93C > T, −210C > T and −2146C > T), but it is not known whether these affect gene expression [30]. These data suggest extensive variation within the human ADRB1 gene, but until it is resequenced in a large enough number of subjects and across different racial groups the exact status of many of these SNPs is uncertain.
Table 1.
The reported SNPs within the coding region of the β1-adrenoceptor gene (ADRB1)
| dbSNP reference* | SNP functionality | Amino acid position | Base change | Position in codon | Amino acid switch | Type of domain |
|---|---|---|---|---|---|---|
| rs1801252 | nonsynonymous | 49 | A > G | 1 | Ser > Gly | Extracellular |
| rs7921133 | synonymous | 105 | G > T | 3 | Leu > leu | Cytoplasmic loop |
| rs238742 | synonymous | 252 | G > A | 3 | Gln > Gln | Cytoplasmic loop |
| rs2773468 | synonymous | 268 | C > G | 3 | Gly > Gly | Cytoplasmic loop |
| rs238741 | nonsynonymous | 318 | C > A | 1 | Arg > Ser | Cytoplasmic loop |
| rs622397 | nonsynonymous | 324 | A > G | 2 | Lys > Arg | Transmembrane |
| rs180897 | nonsynonymous | 343 | G > A | 1 | Ala > Thr | Transmembrane |
| rs189429 | nonsynonymous | 352 | G > T | 3 | Glu > Asp | Extracellular loop |
| rs1801253 | nonsynonymous | 389 | G > C | 1 | Gly > Arg | Cytoplasmic tail |
| rs171170 | nonsynonymous | 400 | G > T | 2 | Arg > leu | Cytoplasmic tail |
| rs365722 | synonymous | 436 | C > T | 3 | Val > Val | Cytoplasmic tail |
| rs238740 | nonsynonymous | 460 | C > A | 3 | Asp > Glu | Cytoplasmic tail |
The current status of each SNP can be checked using the following url: "http://www.w3.org/1999/xlink" xlink:href="http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=# where # is the corresponding rs number.
Table 2.
Species alignment of ADRB1 gene to show highly conserved regions containing the reported nonsynonymous human SNPs from Table 1
β1-AR genetic variation at codon 389
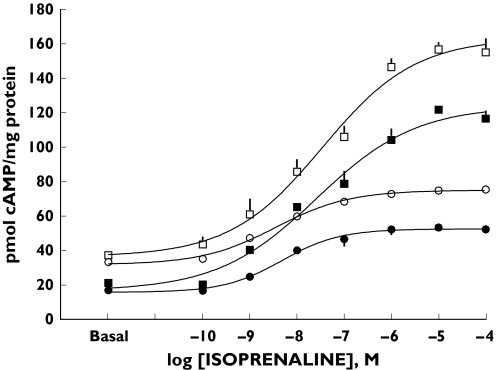
The polymorphism that has aroused most interest has been the C to G switch (1165C > G) that substitutes an arginine for glycine (389R > G) in the C-terminal region of the receptor [25, 26]. Since the substitution lies within the putative G-protein binding domain, it was likely to be functionally important. The 389R residue is also highly conserved across species suggesting that 389R and not 389G is the ancestral or wild type of the receptor (Table 2). In fact, expression in a rodent cell line has demonstrated significant differences in the coupling of the 389R and 389G β1-AR variants to G-proteins in terms of both agonist-promoted receptor binding to Gs and adenylyl cyclase activity [25]. Expression in a human cell line (HEK 293) has produced similar differences in the coupling behaviour of the 389 receptor variants (Figure 1). Although, in HEK cells there were no differences in their constitutive receptor activity (provided they carried the same variant at the 49 position, see below). This may be due to the cell type used (CHW-1102 vs. HEK293) containing different postreceptor components, differences in β1-AR expression density (159 vs. 1100 fmol mg−1) or the postreceptor signalling molecule being studied (AC vs. cAMP) [31]. Nevertheless, the reduced agonist-induced coupling to Gs for the 389G variant explains the reported absence of high affinity binding sites in competition curves performed with Gpp(NH)p [22, 32].
Figure 1 .
Isoprenaline-stimulated cAMP generation by HEK 293 cells expressing GR, SR, GG or SG haplotypes of the β1-AR. Maximum forskolin stimulated cAMP production was not significantly different between the four haplotypes (GR 388 ± 39 (□), SR 402 ± 22 (▪), SG 390 ± 27 (•) and GG 375 ± 32 (○) pmol mg−1 protein). (From [32] with permission)
β1-AR genetic polymorphism at codon 49
The second polymorphism to arouse interest (145G > A) causes nonconservative substitution of glycine for serine (49G > S) in the N-terminus of the β1-AR [25, 33]. By analogy with N-terminal substitutions of the β2-AR, it was expected to affect receptor trafficking. This was confirmed by studying recombinant 49S and 49G receptors and their response to long-term receptor activation (18–24 h). Under these conditions the 49S variant is relatively resistant to agonist-promoted down regulation [34]. The effect of adding cycloheximide to block receptor synthesis suggests that this difference is accounted for by a reduction in the rate of degradation of the 49S receptor after internalization. Immunoblotting of both receptors has shown that the 49S receptor is present in a highly N-glycosylated form that is not found for the 49G variant. This altered glycosylation state may explain the differences in agonist-promoted down regulation, since glycosylation has been reported to affect the down regulation of other G-coupled proteins such as the β2-AR and gastrin releasing peptide receptor [35, 36]. However, the impact of the 49S > G substitution is more extensive, because there are also differences in the rate of desensitization following short-term agonist exposure (10–20 min), with the 49S variant again being relatively resistant vs. the 49G one [37]. Coupling of the receptor variants to adenylyl cyclase is also affected as shown by the constitutive activity of the 49G receptor (see Figure 1). This constitutive activity is almost certainly related mechanistically to its sensitivity to desensitization as well as its increased affinity for β1-adrenoceptor agonists and inverse β1-adrenoceptor antagonists [37].
Haplotype responses
Both the 49S > G and 389R > G polymorphisms are common and native β1-AR function will reflect the variants carried simultaneously at these loci (the receptor haplotype). Individual polymorphisms may act independently to modulate receptor function, although interaction between common polymorphisms is well established, for example, amongst the N-terminal coding variants of the β2-AR [38]. By expressing the four possible haplotypes for the two common β1-AR polymorphisms in HEK 293 cells, it is clear that the four haplotypes demonstrate a functional continuum in terms of their coupling efficiency, basal constitutive activity and agonist-stimulated trafficking [32] (Figure 1). Basal activity was affected by the amino acid at position 49 with the magnitude of differences for isoprenaline evoked cAMP and competition binding between the 389R and 389G receptors similar regardless of 49 genotype. The differences in basal receptor function between the GR and the SR haplotypes persisted throughout the range of agonist-stimulated responses, a pattern found also for the GG and SG haplotypes. This work also confirmed increased desensitization for the 49G and 389R receptors compared with the 49S and 389G receptors, respectively, with enhanced changes for the GR haplotype. This has clear implications for attempts to assign functional roles to these polymorphisms in vivo. Indeed, the focus on single polymorphisms may partly explain the lack of a consistent pharmacogenetic effect of the 49S > G and 389R > G polymorphisms in vivo as discussed below.
Frequencies of β1 AR genetic polymorphisms
Both the 389R > G and 49G > S genetic polymorphisms are common with allele frequencies amongst healthy individuals of 72/0.28 for 389R > G and 0.85/0.15 for 49S > G [25, 26, 39, 40]. Depending on the type of cardiovascular disease, the frequencies differ slightly as outlined below. More striking are the marked ethnic differences, with black African-Americans having a significantly lower frequency of the 389R allele than other ethnic groups: Chinese 74%, Caucasians 72%, Hispanics 67% and African-Americans 58%[39]. Heart failure is more common in African-Americans compared with otherwise similar cohorts of Caucasians, and the 389R > G polymorphism may provide a genetic basis for this increased incidence. A separate study has confirmed ethnic differences for the 389R > G polymorphism, but failed to identify similar ethnic differences for the 49S > G polymorphism between Caucasians, Chinese and African-Americans [41]. A novel polymorphism in the 5′ flanking region of the β1-AR gene (−2146T > C) exists in almost complete linkage disequilibrium with the 49S > G polymorphism with reported frequencies for both mutations of 0.108 for controls, 0.163 for IDCM patients and 0.130 for CHD patients [30]. There is also data suggesting linkage disequilibrium between the 49S > G and 389R > G polymorphisms. A study that genotyped almost 700 women found none with the 49G/389G haplotype, despite it being the expected haplotype for 52 of them [42].
Associations of β1-AR polymorphisms with cardiac function
Despite a consensus in the literature on the functional implications of the 389R > G and 49S > G polymorphisms for the β1-AR expressed in vitro, publications looking at the in vivo impact of the polymorphism in humans have been more variable. Several studies have investigated the effects of these polymorphisms on resting haemodynamics and the incidence of hypertension. A Scandinavian study using genotype-discordant sibs, reported that sibs homozygous for the 389R allele had significantly higher diastolic blood pressure and heart rates than siblings carrying either one or two copies of the 389G allele. A case-control study from the same group also found that 389R homozygotes were more likely to be hypertensive with an age and body mass index adjusted odds ratio for hypertension of 1.9 [33]. Of note, there was no effect of the 49S > G polymorphism on blood pressure or heart rate in this study. Humma et al.[43] also found that 389R homozygotes had higher blood pressure and heart rate than heterozygotes, although this only applied to the Caucasian subjects in their mixed cohort. Other groups have failed to confirm an effect on resting BP or heat rate from the 389R allele [44–46], and in a large cohort of Chinese and Japanese subjects it was the 49S > G polymorphism that independently affected heart rate and not the 389R > G one [44].
From the in vitro behaviour of the dose–response curves for the 389R and 389G receptors it might be expected that in vivo differences would be more obvious under high rates of sympathetic activation (and hence receptor occupancy). However, both studies using exercise-induced heart rates or other markers of β1-AR activation (QS2c and plasma renin release) failed to find an effect of the 389R > G genotype in normal healthy volunteers [39, 40]. Disease status may be a more reliable way of separating the effect of genotype. In the failing myocardium, the loss of β1-AR coupling efficiency may impact more on the 389G receptor with its already lower coupling efficiency. Two clinical studies support this. Wagoner et al.[47] looked at β1-AR polymorphisms and exercise responses in 263 patients with heart failure and found that despite matched left ventricular function 389R homozygotes had higher peak VO2 and exercise capacity than 389G homozygotes; heterozygotes were intermediate suggesting a gene dose effect. Analyzing the results as a 389/49 haplotype showed a graded response across the five haplotypes with homozygous 389G/49S having the lowest and homozygous 389R/49G carriers the highest VO2 and exercise capacity. These physiological variables appear to be more sensitive to β1-AR genotype since peak heart rate or blood pressure was not affected by genotype.
Another study has reported an effect of the 389R > G genotype on LV mass. In a group of patients attending a renal clinic 389G homozygotes had significantly higher raw LV mass, LV mass index and LV mass indexed-to-height compared to carriers of the 389R allele. This effect persisted even when patients on β-adrenoceptor blockers or renal replacement therapy were removed from the analysis. The 389G homozygotes did have slightly higher blood pressures (∼2 mmHg) but this cannot explain their substantially heavier ventricles [48]. It appears that the 389 allele can affect myocardial cell growth directly, but here it is the 389G and not 389R receptor that confers a gain-of-function.
β1-AR polymorphisms and responses to antagonists
The discovery of polymorphisms within the β1-AR has raised considerable interest in the part they might play in determining the clinical response to β1-AR antagonists. The first study to look for a pharmacogenetic effect analyzed retrospectively the response of a cohort of 147 untreated hypertensive patients randomly assigned to β1-AR blockade with either bisoprolol or atenolol. In this study, the 389R > G genotype had no influence on the blood pressure or heart rate responses measured 4 weeks from baseline [45]. Similar findings were reported in patients with heart failure recruited into the MERIT-HF trial and treated with metoprolol CR/XL [49]. The 389R > G genotype influenced neither the reduction in heart achieved nor the mortality/morbidity benefit seen in patients randomized to treatment with metoprolol. Another outcome study of 479 patients with early heart failure (mean LVEF <45%) treated with either bisoprolol or carvedilol found that neither the 389R > G or 49S > G polymorphisms affected the event-free survival [50].
In contrast to these findings, small-scale prospective trials have generally reported a highly significant pharmacogenetic effect. Johnston et al.[51] found greater reductions in 24 h and daytime diastolic blood pressure in 389R homozygotes compared with 389G carriers. Inclusion of the 49S > G polymorphism actually showed a gradation to the hypotensive response, which was greatest for double homozygotes (389R/49S) and negligible in double carriers of the G alleles (389G/49G). A multivariate analysis further suggested 389R and 49S as the only independent predictors of daytime diastolic blood pressures. Two other studies have reported on the effect of atenolol or metoprolol on basal and exercise-induced blood pressure and heart rate. Sofowora et al.[46] reported marked differences in subjects who are 389R homozygotes vs. 389G homogygotes: the mean fall in systolic pressures were, respectively, ∼9 and ∼0 mmHg. Interestingly, there was no difference in the effect on basal heart rate and diastolic BP or the change in any of these variables with exercise. The study by Liu et al.[52] reported similar, but smaller, differences in systolic BP reductions after metoprolol in 389R vs. 389G homozygotes. This study also found no difference in diastolic BP reductions, although reductions in heart rate were different both at baseline and after exercise in the two groups. Since β1-AR antagonists lower blood pressure by different mechanisms after acute and chronic dosing, it is notable that both of these studies looked at responses to acute dosing with a β1-AR antagonist. Finally, in a cohort of heart failure patients dose titrated with carvedilol, only 389R homozygotes had a benefit in terms of LVEF: the mean rise was 8.7%vs. < 1% in 389G homozygotes [53]. The differences in trial design and use of dose titration may explain why a pharmacogenetic effect of the 389R > G polymorphism in particular was seen in these studies but not in the earlier retrospectives ones.
Ex vivo effect of β1-AR polymorphisms
A complementary strategy to define the functional importance of receptor polymorphisms is the use of human cells or tissues expressing native β1-ARs of a particular genotype. A tissue that has been particularly useful for this purpose has been the atrial appendage; which can be recovered relatively easily from patients undergoing cardiac surgery and allows direct assessment of myocyte function ex vivo. Hence, atrial appendages recovered from homozygous 389R patients are reported as having increased sensitivity to noradrenaline compared with 389G homozygotes, although maximal contractile responses were intact [54]. This behaviour contrasts with experience in transgenic cell lines where both the sensitivity and maximal responses differed in 389R vs. 389G receptors (see Figure 1). However, a further report by Molennar et al.[55] has failed to show any ex vivo differences at all in the responses of atrial appendages across both the 389R > G and 49G > S polymorphisms. It is not clear whether patient selection or other technical differences between these studies explains this conflict.
Since β1-ARs are also endogenously expressed on adipocytes this has been an obvious cell type to study. The effect of the 389R > G polymorphism on human fat cell lipolysis was examined in 298 subjects who covered a wide range of body mass indices [56]. Using noradrenaline as the stimulus for glycerol release, this group failed to detect differences in the responses for subjects carrying the 389R vs. the 389G receptor. Analyzing either all subjects together or as subgroups based on BMI and gender did not affect the result. The frequency distribution of the 389R > G polymorphism was also similar between lean and obese subjects implying that the 389R > G polymorphism does not influence body mass. However, this interpretation may have been confounded because antilipolytic α-effects were not taken into account and adipocytes express β2 and β3-ARs that can also mediate lipolysis. So in summary, the ex vivo study of native β1-ARs has not provided any more clarity about the functional impact of the β1-AR receptor polymorphisms than the in vivo trials.
Disease risk for β1-AR polymorphisms
Another strategy is to determine whether the β1-AR genotype shows association with some aspect of cardiovascular disease such as its prevalence, severity or progression. This approach has been applied to heart failure and ischaemic heart disease, although results have been generally disappointing. For example, in the Cardigene population of clinically well-characterized patients with idiopathic dilated cardiomypoathy (IDCM), the 389R > G allele frequency was similar to age and sex matched controls [57]. This is surprising given that patients with heart failure have high circulating catecholamine concentrations, the in vitro effect of the polymorphism is substantial, there is well described down-regulation of the β1-AR in heart failure and there is clear therapeutic benefit of β1-AR blockade. In another study, heart failure patients carrying the 49G allele were associated with decreased mortality risk when followed for 5 years, but again they had a similar incidence of the variant compared with controls [58]. What is lacking to date is a study that addresses haplotype response rather than the 389R > G and 49S > G taken in isolation. Their impact also appears to be modulated by other adrenoceptor polymorphisms, since the α2c-AR acts synergistically with the 389R allele to increase the risk of heart failure especially amongst Black subjects [59].
The β1-AR has been extensively implicated in the progression of ischaemic heart disease and myocardial infarction. The reduced function of the 389G variant in vitro also effectively produces a naturally β1-AR blocked form of the β1-AR suggesting it may be a protective allele. Yet, there was no evidence for this in the longitudinal data set from the WOSCOPS study [60]. Here men with a coronary event were matched for age and smoking status with two control subjects from the same cohort, and 389R > G genotype frequencies compared in the two groups. There were no differences and to date just one study has reported a protective effect of the 389G allele [43]. Since this was actually a protective effect against ventricular arrhythmias in patients with IDCM, it seems unlikely that it can be explained by an influence on IHD alone.
The last association study involved a cohort of 931 Caucasian women genotyped for 389R > G polymorphism, which reported an association of the 389R allele with greater body weight and body mass index [44]. The higher frequency of the 389R variant in obese subjects was hypothesized to lead to increased lipolysis and visceral fat deposition, but is not supported by the ex vivo findings in adipocytes [45].
Use of transgenic animals
An obvious genomic tool to use to explore the functional significance of the polymorphisms is targeted transgenesis. This was first reported by Mialat-Perez et al.[46] utilizing transgenic mice to over express either 389R or 389G β1-ARs in ventricular tissues. Hearts from young 389R mice possessed enhanced receptor function and contractility compared with 389G hearts. Presumably reflecting the chronic enhanced adrenergic drive possible through the 389R receptor, the differences in contractile responses to dobutamine reversed in older mice. The protective loss of β1-AR density was also significantly lower in 389R hearts, although they were more sensitive to the effects on contractility of receptor blockade with propranolol. These results may not be directly applicable to human hearts, which have an intrinsically lower adrenergic tone and level of β1-AR expression compared with rodents. Nevertheless, the same authors have reported that clinical benefit from β1-AR blockade of patients with heart failure is more likely in carriers of the 389R receptor. Hence, transgenic models based on the 49S > G polymorphism or haplotype extremes of 389R > G/49S > G could provide further important clues about the role of β1-AR pharmacogenetics in human heart failure.
Conclusions
The search for functional evidence of the importance of the two common β1-AR polymorphisms, 389R > G and 49S > G has suggested substantial effects both in and ex vivo. But a functional comparison especially outside of transgenic cell lines has been very inconsistent, and this lack of consistency needs to be addressed. Nevertheless, it seems likely that these polymorphisms will impact on clinical practice, both in terms of predicting cardiovascular disease risk as well as in the pharmacological management of cardiac disease with β1-AR antagonists.
Acknowledgments
Competing interests: None declared.
References
- 1.Ablad B, Carlsson B, Carlsson E, Dahlof C, Ek L, Hultberg E. Cardiac effects of β-AR receptor antagonists. Adv Cardiol. 1974;12:290–302. doi: 10.1159/000395473. [DOI] [PubMed] [Google Scholar]
- 2.Insel PA, Snavely MD. Catecholamines and the kidney. receptors and renal function. Ann Rev Physiol. 1981;43:625–36. doi: 10.1146/annurev.ph.43.030181.003205. [DOI] [PubMed] [Google Scholar]
- 3.Fain J, Garcia-Sanz J. Adrenergic regulation of adipocytes metabolism. J Lipid Res. 1983;24:945–86. [PubMed] [Google Scholar]
- 4. http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=153&choosers=coding.
- 5.Ahlquist RP. A study of the adrenotropic responses. Am J Physiol. 1948;53:586–600. doi: 10.1152/ajplegacy.1948.153.3.586. [DOI] [PubMed] [Google Scholar]
- 6.Lands AM, Arnold A, McAuliff JP, Luduena FP, Brown TG., Jr Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967;214:597–8. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- 7.Hausdorf W, Caron M, Lefkowitz RJ. Turning off the signal: desensitisation of β-AR function. FASEB J. 1990;4:2881–9. [PubMed] [Google Scholar]
- 8.Hamm H. The many faces of G-protein signalling. J Biol Chem. 1998;273:669–72. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- 9.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC. Decreased catecholamine sensitivity and β-AR density in failing human hearts. N Engl J Med. 1992;307:205–11. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 10.Frielle T, Collins S, Daniel K, Caron M, Lefkowitz RJ, Kobilka B. Cloning of the cDNA for the human β-1AR. PNAS. 1987;84:7920–4. doi: 10.1073/pnas.84.22.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoehe M, Berrettini W, Leppert M, Lalouel JM, Byerley W, Gersham E, White R. Genetic mapping of adrenergic receptor genes. Am J Hum Genet. 1989;45:A143. [Google Scholar]
- 12.Frielle T, Daniel K, Caron M, Lefkowitz RJ. Structural basis of β-AR subtype specificity studied with chimeric receptors. PNAS. 1988;85:9494–8. doi: 10.1073/pnas.85.24.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green SA, Liggett SB. A proline rich region of the third intracellular loop imparts phenotypic β-1 versus β-2 AR coupling and sequestration. J Biol Chem. 1994;269:26215–9. [PubMed] [Google Scholar]
- 14.Lattion AL, Abuin L, Nenniger-Tosato M, Cotecchia S. Constitutively active mutants of the β-1 AR. FEBS Lett. 1999;457:302–6. doi: 10.1016/s0014-5793(99)01064-9. [DOI] [PubMed] [Google Scholar]
- 15.Rousseau G, Nantel F, Bouvier M. Distinct receptor domains determine subtype specific coupling and desensitisation phenotypes for human β-1 and β-2 AR. Mol Pharmacol. 1996;49:752–60. [PubMed] [Google Scholar]
- 16.Jingi HE, Jianguo XU, Castleberry AM, Lall AG, Hall RA. Glycosylation of the β-1AR's regulates receptor surface expression and dimerisation. Biochem Biophys Res Comm. 2002;297:565–72. doi: 10.1016/s0006-291x(02)02259-3. [DOI] [PubMed] [Google Scholar]
- 17.Dayer P, Merier G, Perrenoud JJ, Marmy A, Leemnri T. Interindividual pharmacokinetics and pharmacodynamic variability of different β-blockers. J Cardiovasc Pharmacol. 1986;8:20–4. doi: 10.1097/00005344-198608006-00005. [DOI] [PubMed] [Google Scholar]
- 18.Martinsson A, Lindwall K, Melcher A, Hjemdahl P. Beta-adrenergic receptor responsiveness to isoprenaline in humans: concentration effect as compared with dose effect, evaluation and influence of autonomic reflexes. Br J Clin Pharmacol. 1989;28:83–94. doi: 10.1111/j.1365-2125.1989.tb03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liggett SB, Shah SD, Cryer PE. Human tissue adrenergic receptors are not predictive of responses to epinephrine in vivo. Am J Physiol. 1989;256:600–9. doi: 10.1152/ajpendo.1989.256.5.E600. [DOI] [PubMed] [Google Scholar]
- 20.Van Campen LC, Visser FC, Visser CA. Ejection fraction improvement by beta-blocker treatment in patients with heart failure: an analysis of studies published in the literature. J Cardiovasc Pharmacol. 1998;32:31–5. doi: 10.1097/00005344-199800003-00006. [DOI] [PubMed] [Google Scholar]
- 21.Liggett SB. Pharmacogenetics of β-1 and β-2 AR’s. Pharmacology. 2000;61:167–73. doi: 10.1159/000028397. [DOI] [PubMed] [Google Scholar]
- 22.Bristow MR, Hergberger RE, Port JD, Rasmussen R. β-1 AR and β-2 AR mediated adenylate cyclase in non-failing and failing human ventricular myocardium. Mol Pharmacol. 1989;35:295–303. [PubMed] [Google Scholar]
- 23.Leineweber K, Brodde OE. Beta2-adrenoceptor polymorphisms: relation between in vitro and in vivo phenotypes. Life Sci. 2004;74:2803–14. doi: 10.1016/j.lfs.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 24.Berrettini W, Hoehe M. polymorphism of the β-1 AR detected with Bgl I. Nucl Acids Res. 1988;16:7754. doi: 10.1093/nar/16.15.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maqbool A, Hall AS, Ball SG, Balmforth AJ. Common polymorphisms of beta1-adrenoceptor: identification and rapid screening assay. Lancet. 1999;353:897. doi: 10.1016/s0140-6736(99)00549-8. [DOI] [PubMed] [Google Scholar]
- 26.Mason DA, Moore JD, Green SA, Liggett SB. A gain-of-function polymorphism in a G-protein coupling domain of the human beta 1-adrenergic receptor. J Biol Chem. 1999;274:12670–4. doi: 10.1074/jbc.274.18.12670. [DOI] [PubMed] [Google Scholar]
- 27.Tesson F, Charron P, Peuchmaurd M, Nicaud V, Cambien F, Tiret L, Poirier O, Desnos M, Jullieres Y, Amouyel P, Roizes G, Dorent R, Schwartz K, Komajda M. Cardigene Group. A characterisation of a unique gene variant in the β-1 AR gene and evaluation of its role in IDCM. J Mol Cell Cardiol. 1999;31:1025–32. doi: 10.1006/jmcc.1999.0947. [DOI] [PubMed] [Google Scholar]
- 28.Borjesson M, Magnusson Y, Hjalmarson A, Andersson B. A novel polymorphism in the gene coding for the β1-adrenergic receptor associated with survival in patients with heart failure. Eur Heart J. 2000;21:1853–8. doi: 10.1053/euhj.1999.1994. [DOI] [PubMed] [Google Scholar]
- 29.Podlowski S, Wengel K, Luther H, Muller J, Bramlage P, Baumann G, Felix S, Speer A, Hetzer R, Kopke K, Hoehe M, Wallukat G. β-1 adrenoceptor gene variations: a role in IDCM. J Mol Med. 2000;78:87–93. doi: 10.1007/s001090000080. [DOI] [PubMed] [Google Scholar]
- 30.Wenzel K, Felix SB, Bauer D, Heere P, Flachmeier C, Podlowski S, Kopke K, Hoehe MR. Novel variants in the 3Kb of 5′ UTR of the α-1 adrenergic receptor gene (−93 C>T-210 C>T and –2146 T>C): -2146C homozygotes present in patients with idiopathic dilated cardiomyopathy and coronary heart disease. Human Mutation. 2000. Mutation and Polymorphism report #187. [DOI] [PubMed]
- 31.Whaley BS, Yuan N, Birnbaumer L, Clark RB, Barber R. Differential expression of the beta-adrenergic receptor modifies agonist stimulation of adenylyl cyclase: a quantitative evaluation. Mol Pharmacol. 1994;45:481–9. [PubMed] [Google Scholar]
- 32.Sandilands AJ, Yeo G, Brown MJ, O'Shaughnessy KM. Functional responses of human β-1 adrenoceptors with defined haplotypes for the common 389R>G and 49S>G polymorphisms. Pharmacogenetics. 2004;14:343–9. doi: 10.1097/00008571-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Bengtsson K, Melander O, Orho-Melander M, Lindbald U, Ranstam J, Ranstam L, Groop L. Polymorphism in the â1-adrenergic receptor gene and hypertension. Circulation. 2001;104:187–90. doi: 10.1161/01.cir.104.2.187. [DOI] [PubMed] [Google Scholar]
- 34.Rathz DA, Brown KA, Kramer LA, Liggett SB. Amino acid polymorphisms of the human β1-adrenergic receptor affect agonist-promoted trafficking. J Cardiovascular Pharmacol. 2002;39:155–60. doi: 10.1097/00005344-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Rands E, Canelore MR, Cheung AH. Mutational analysis of β-AR glycosylation. J Biol Chem. 1990;265:10759–64. [PubMed] [Google Scholar]
- 36.Benya RV, Kusui T, Katsuno T. Glycosylation of the gastrin releasing paptide receptor and its effect on expression, G protein coupling and receptor modulatory processes. Mol Pharmacol. 2000;58:1490–501. doi: 10.1124/mol.58.6.1490. [DOI] [PubMed] [Google Scholar]
- 37.Levin MC, Marullo S, Muntaner O, Andersson B, Magnusson Y. The myocardium-protective Gly-49 variant of the α-1 adrenergic receptor exhibits constitutive activity and increased desensitisation and down-regulation. J Biol Chem. 2002;277:30429–35. doi: 10.1074/jbc.M200681200. [DOI] [PubMed] [Google Scholar]
- 38.Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human beta-2 adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33:9414–9. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- 39.Xie HG, Dishy V, Sofowora G, Kim RB, Landau R, Smiley RM, Zhou HH, Wood AJ, Harris P, Stein CM. Arg389Gly α-1 adrenergic receptor polymorphism varies in frequency among different ethnic groups but does not alter response in vivo. Pharmacogenetics. 2001;11:191–7. doi: 10.1097/00008571-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Buscher R, Belger H, Eilmes KJ, Tellkamp R, Radke J, Dhein S, Hoyer PF, Michel MC, Insel PA, Brodde OE. In vivo studies do not support a major functional role for the Gly389Arg β-1 AR polymorphisms in humans. Pharmacogenetics. 2001;11:199–205. doi: 10.1097/00008571-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Moore DJ, Mason DA, Green SA, Hsu J, Liggett SB. Racial differences in the frequencies of cardiac β-1 AR receptor polymorphisms. Analysis of c145A>G and c1165G>C. Human Mutation. 1999. Human mutation report # 65. [DOI] [PubMed]
- 42.Terra SG, McGorray SP, Wallace MR, Pepine CJ, Johnson JA. Linkage disequilibrium of common α-1 adrenergic receptor polymorphisms. Clin Pharmacol Ther. 2002:Abstract TPII-103. [Google Scholar]
- 43.Humma LM, Puckett BJ, Richardson HE, Terra SG, Andrisin TE, Lejeune BL, Wallace MR, Lewis JF, McNamara M, Picoult-Newberg L, Pepine CJ, Johnson JA. Effects of β-1 AR genetic polymorphisms on resting haemodynamics in patients undergoing diagnostic testing fir ischaemia. Am J Cardiol. 2001;88:1034–7. doi: 10.1016/s0002-9149(01)01986-5. [DOI] [PubMed] [Google Scholar]
- 44.Ranade K, Jorgenson EH-H, Sheu W, Pei D, Hsiung CA, Chiang F, Chen YI, Pratt R, Olshen RA, Curb D, Cox DR, Botstein D, Risch N. A polymorphism in the β1 adrenergic receptor is associated with resting heart rate. Am J Hum Genet. 2002;70:935–42. doi: 10.1086/339621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Shaughnessy KM, Fu B, Dickerson C, Thurston D, Brown MJ. The gain of function variant (R389G) of the β-1 AR does not influence blood pressure or response to β-blockade in hypertensive subjects. Clin Sci (Lond) 2000;99:233–8. [PubMed] [Google Scholar]
- 46.Sofowora GG, Dishy V, Muszkat M, Xie G, Kim RB, Harris PA, Prasad HC, Byrne DW, Nair UB, Wood AJ, Stein CM. A common α-1 adrenergic receptor polymorphism (Arg389Gly) affects blood pressure response to α-blockade. Clin Pharmacol Ther. 2003;73:366–71. doi: 10.1016/s0009-9236(02)17734-4. [DOI] [PubMed] [Google Scholar]
- 47.Wagoner LE, Craft LL, Zengel P, McGuire N, Rathz DA, Dorn GW, Liggett SB. Polymorphisms of the α-1 adrenergic receptor predict exercise capacity in heart failure. Am Heart J. 2002;144:840–6. doi: 10.1067/mhj.2002.125325. [DOI] [PubMed] [Google Scholar]
- 48.Stanton T, Inglis GC, Padmanabhan S, Dominiczak AF, Jardine AG, Connell JMC. Variation at the α-1 adrenergic receptor gene locus affects left ventricular mass in renal failure. J Nephrol. 2002;15:512–8. [PubMed] [Google Scholar]
- 49.White HL, de Boer RA, Maqbool A, Greenwood D, van Veldhuisen DJ, Cuthbert R, Ball SG, Hall AS, Balmforth AJ. An evaluation of the β-1 AR Arg389Gly polymorphism in individuals with heart failure: a MERIT-HF sub study. Eur J Heart Fail. 2003;5:463–8. doi: 10.1016/s1388-9842(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 50.Postava L, Mahlab D, Holubkov R, Janosko K, Palmer A, MacGowan G, Murali S, London B, McNamara DM. β-1 AR and β-2 AR polymorphisms and heart failure survival: interaction with β-blockade. Circulation. 2002;105:Abstract 3019. [Google Scholar]
- 51.Johnson JA, Zineh I, Puckett BJ, McGorray SP, Yarandi HN, Pauly DF. α-1 AR polymorphisms and antihypertensive response to metoprolol. Clin Pharmacol Ther. 2003;74:44–52. doi: 10.1016/S0009-9236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Liu ZQ, Tan ZR, Chen XP, Wang LS, Zhou G, Zhou HH. Gly389Arg polymorphism of beta 1-adrenergic receptor is associated with the cardiovascular response to metoprolol. Clin Pharmacol Ther. 2003;74:372–9. doi: 10.1016/S0009-9236(03)00224-8. [DOI] [PubMed] [Google Scholar]
- 53.Mialet Perez JM, Rathz DA, Petrashevskaya NN, Hahn HS, Wagoner LE, Scwartz A, Dorn IIGW, Liggett SB. β-1 AR polymorphisms confer differential function and predisposition to heart failure. Nature Med. 2003;9:1300–5. doi: 10.1038/nm930. [DOI] [PubMed] [Google Scholar]
- 54.Sandilands AJ, O'Shaughnessy KM, Brown MJ. Greater inotropic and cyclic AMP responses evoked by noradrenaline through Arg389 β-1 adrenoceptors versus Gly389 β-1 adrenoceptors in isolated human atrial myocardium. Br J Pharmacol. 2003;138:386–92. doi: 10.1038/sj.bjp.0705030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molenaar P, Rabnott G, Yang I, Fong KM, Savarimuthu SM, West MJ, Russell FD. Conservation of the cardiostimulant effects of norepinephrine across Ser49Gly and Gly389Arg α-1 adrenergic receptor polymorphisms in human right atrium in vitro. J Am Col Cardiol. 2002;40:1275–82. doi: 10.1016/s0735-1097(02)02137-x. [DOI] [PubMed] [Google Scholar]
- 56.Ryden M, Hoffstedt J, Erikkson P, Bringman S, Arner P. The Arg 389 Gly β-1 adrenergic receptor gene polymorphism and human fat cell lipolysis. Int J Obes Relat Metab Disord. 2001;25:1599–603. doi: 10.1038/sj.ijo.0801815. [DOI] [PubMed] [Google Scholar]
- 57.Tesson F, Charron P, Peuchmaurd M, Nicaud V, Cambien F, Tiret L, Poirier O, Desnos M, Jullieres Y, Amouyel P, Roizes G, Dorent R, Schwartz K, Komajda M Cardigene Group. A characterisation of a unique gene variant in the β-1 AR gene and evaluation of its role in IDCM. J Mol Cell Cardiol. 1999;31:1025–32. doi: 10.1006/jmcc.1999.0947. [DOI] [PubMed] [Google Scholar]
- 58.Borjesson M, Magnusson Y, Hjalmarson A, Andersson B. A novel polymorphism in the gene coding for the β1-adrenergic receptor associated with survival in patients with heart failure. Eur Heart J. 2000;21:1853–8. doi: 10.1053/euhj.1999.1994. [DOI] [PubMed] [Google Scholar]
- 59.Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of the α-1 and α2c adrenergic receptors and the risk of heart failure. N Engl J Med. 2002;347:1135–42. doi: 10.1056/NEJMoa020803. [DOI] [PubMed] [Google Scholar]
- 60.White HL, Maqbool A, McMahon AD, Yates L, Ball SG, Hall AS, Balmforth AJ. An evaluation of the β-1 AR Arg389Gly polymorphism in individuals at risk of coronary events. Eur Heart J. 2002;23:1087–92. doi: 10.1053/euhj.2001.3037. [DOI] [PubMed] [Google Scholar]
- 61.Iwai C, Akita H, Shiga N, Takai E, Miyamoto Y, Shimizu M, Kawai H, Takarada A, Kajiya T, Yokoyama M. Suppressive effect of the Gly389 allele of the β-1 AR gene on the occurrence of ventricular tachycardia in dilated cardiomyopathy. Circulation J. 2002;66:723–8. doi: 10.1253/circj.66.723. [DOI] [PubMed] [Google Scholar]
- 62.Dionne IJ, Garant MJ, Pollin TI, Lewis DG, Shuldiner AR, Poehlman ET. Association between obesity and a polymorphism in the α-1 adrenergic receptor gene (Gly389Arg ADRB1) in Caucasian women. Int J Obesity. 2002;26:633–9. doi: 10.1038/sj.ijo.0801971. [DOI] [PubMed] [Google Scholar]