Abstract
Aim
The aim of this study was to investigate the effect of two common herbal medicines, ginkgo and ginger, on the pharmacokinetics and pharmacodynamics of warfarin and the independent effect of these herbs on clotting status.
Methods
This was an open label, three-way crossover randomized study in 12 healthy male subjects, who received a single 25 mg dose of warfarin alone or after 7 days pretreatment with recommended doses of ginkgo or ginger from herbal medicine products of known quality. Dosing with ginkgo or ginger was continued for 7 days after administration of the warfarin dose. Platelet aggregation, international normalized ratio (INR) of prothrombin time, warfarin enantiomer protein binding, warfarin enantiomer concentrations in plasma and S-7-hydroxywarfarin concentration in urine were measured. Statistical comparisons were made using anova and the 90% confidence intervals (CIs) of the ratio of log transformed parameters are reported.
Results
INR and platelet aggregation were not affected by administration of ginkgo or ginger alone. The mean (95% CI) apparent clearances of S-warfarin after warfarin alone, with ginkgo or ginger were 189 (167–210) ml h−1, 200 (173–227) ml h−1 and 201 (171–231) ml h−1, respectively. The respective apparent clearances of R-warfarin were 127 (106–149) ml h−1, 126 (111–141) ml h−1 and 131 (106–156) ml h−1. The mean ratio (90% CI) of apparent clearance for S-warfarin was 1.05 (0.98–1.21) and for R-warfarin was 1.00 (0.93–1.08) when coadministered with ginkgo. The mean ratio (90% CI) of AUC0−168 of INR was 0.93 (0.81–1.05) when coadministered with ginkgo. The mean ratio (90% CI) of apparent clearance for S-warfarin was 1.05 (0.97–1.13) and for R-warfarin was 1.02 (0.95–1.10) when coadministered with ginger. The mean ratio (90% CI) of AUC0−168 of INR was 1.01 (0.93–1.15) when coadministered with ginger. The mean ratio (90% CI) for S-7-hydroxywarfarin urinary excretion rate was 1.07 (0.85–1.32) for ginkgo treatment, and 1.00 (0.81–1.23) for ginger coadministration suggesting these herbs did not affect CYP2C9 activity. Ginkgo and ginger did not affect the apparent volumes of distribution or protein binding of either S-warfarin or R-warfarin.
Conclusions
Ginkgo and ginger at recommended doses do not significantly affect clotting status, the pharmacokinetics or pharmacodynamics of warfarin in healthy subjects.
Keywords: anticoagulants, herb–drug interactions, ginkgo, ginger, warfarin
Introduction
The anticoagulant warfarin is a commonly prescribed medication which has a narrow therapeutic index and displays high inter- and intrasubject variability in response. In the community there is also widespread, often unreported, self-medication with a range of herbal medicines. The opportunity for potentially life-threatening interactions between herbal medications and warfarin is thus high [1]. Ginkgo (Ginkgo biloba) is one such herbal medicine which is commonly used as the ginkgo extract EGb 761 for promoting and maintaining mental alertness, concentration and focus as well as for a wide range of other indications [2, 3]. However, relatively little is know about ginkgo–drug interactions. A recent review article [1] identified one case report of an interaction between ginkgo and warfarin and four case reports of spontaneous bleeding associated with the use of ginkgo alone attributed to possible effects on platelet function. In vitro studies indicate that constituents of Ginkgo biloba (ginkgolic acids I and II) inhibit drug metabolizing enzymes including CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4 [4]. In contrast, administration of ginkgo extracts to rats for 4 weeks reportedly reduced the hypotensive effect of nicardipine (which is metabolized by CYP3A2) and induced hepatic CYP2B1/2, CYP3A1 and CYP3A2 mRNA [5]. In vitro animal and in vivo clinical studies have investigated the effect of ginkgo extracts on platelet aggregation and coagulation, but the results have been conflicting [6–9].
Ginger (Zingiber officinale) is a herbal medicine taken for a variety of indications including the symptomatic relief of motion sickness and inflammation [10], but few drug interaction studies have been undertaken with this herb. Several in vitro studies have demonstrated that platelet aggregation is inhibited by ginger extracts [11–15]. Furthermore, a single dose (10 g) of powdered ginger inhibited platelet aggregation in patients [16].
The aim of the present study was to investigate the possible herb–drug interactions between warfarin and these two widely used herbal medicines and their independent effects on clotting status.
Materials and methods
Herbal medicines
The herbal medicine products were selected after high-performance thin-layer chromatography (HPTLC) was used to characterize the constituents of several commercially available herbal medicine products containing ginkgo and ginger, according to methods described in the ginkgo monograph of the People's Republic of China Pharmacopeia (English Edition, 2000) and the ginger monograph of the British Pharmacopeia (2001), respectively. The proprietary products selected for this study were Tavonin™ (Ginkgo biloba, each tablet containing the standardized dry extract, EGb761, equivalent to 2 g of Ginkgo biloba leaf, 9.6 mg of ginkgo flavonglycosides, 2.4 mg of ginkgolides and bilobalide, Batch 6250202, Dr Willmar Schwabe GmbH & Co. Germany, marketed by Flordis™, Epping, NSW, Australia) and Blackmores Travel Calm Ginger (Zingiber officinale, each capsule containing extract equivalent to 0.4 g of ginger rhizome powder, Batch 103863, Blackmores Ltd, Balgowlah, NSW, Australia).
Subjects
Twelve healthy male subjects were recruited into three study groups. Subjects were aged 20–36 years, and were within 15% of ideal body weight for height and build. The study group consisted of six Caucasian and six Asian subjects. All subjects were nonsmokers and were selected on the basis of medical history, physical examination and clinical laboratory test results (including INR, platelet aggregation, creatinine, bilirubin, albumin and total protein). Subjects with current or past medical conditions that might affect the pharmacokinetic or pharmacodynamic response to warfarin were excluded from the study. Furthermore, subjects had not taken any medication for at least 2 weeks before commencing the study. Power calculations indicated that 12 subjects in a crossover study would provide an 80% chance of detecting a 20% difference in the AUC0–∞ of S-warfarin. The same study design has been employed to investigate the interaction between warfarin and the herbs St John's wort and ginseng [17]. All participants gave written informed consent before entering the study. The study was approved by the St Vincent's Hospital Human Research Ethics Committee and the Human Ethics Committee of the University of Sydney.
Study design
A randomized, open label, three-treatment, three-period, three-sequence, crossover study was conducted with at least a 14-day washout between study periods. A single 25 mg dose of rac-warfarin (Coumadin™, 5 × 5 mg tablets, Boots HealthCare Australia Pty Ltd, North Ryde, NSW, Australia) was administered to each subject with and without pretreatment with multiple doses of either ginkgo (two tablets, 3× day−1 for 1 week) or ginger (three tablets, 3× day−1 for 1 week). Dosing of ginkgo or ginger was continued for a further 1 week after warfarin administration. Blood samples were collected via an indwelling cannula or by individual venipuncture into both sodium citrate and EDTA tubes. Sampling times in relation to warfarin dosing were: −48, −24, 0, 1, 2, 4, 8, 12, 24, 48, 72, 96, 120, 144 and 168 h. Whole blood was used to measure platelet aggregation, and plasma was harvested by centrifugation (at 1500 g for 10 min) to determine the INR. A portion of plasma was stored frozen until the time of drug concentration analysis. Urine was collected before and after administration of warfarin for 3 days. The volume of urine was recorded and a portion was stored frozen for subsequent analysis.
Analytical techniques
The concentrations of S-warfarin and R-warfarin in plasma and S-7-hydroxywarfarin in urine were determined using a modified version of the HPLC assay by Naidong et al.[18] which employs a chiral HPLC column (Silica-bonded β-cyclodextrin, Cyclobond™, Astec, Alltech Associates Australia Pty Ltd, Baulkham Hills, NSW, Australia) with fluorescence detection. The mobile phase comprised acetonitrile: methanol: triethylamine: glacial acetic acid (95 : 5 : 0.2 : 0.3, v/v/v/v) at a flow rate of 1 ml min−1. Measurements were made using a Shimadzu RF 535 fluorescence detector (excitation wavelength 310 nm, emission wavelength 400 nm) (Shimadzu Scientific Instruments (Oceania) Pty Ltd, Rydalmere, NSW, Australia). The assay was linear for S-warfarin and R-warfarin over the range 20 ng ml−1 to 2500 ng ml−1 in plasma and was linear for S-7-hydroxywarfarin over the range of 33 ng ml−1 to 1650 ng ml−1 in urine. The precision of the assay was less than 15% as indicated by the percent coefficient of variation and the accuracy of the assay were within 15% of the actual value for S-warfarin, R-warfarin and S-7-hydroxywarfarin [17].
Plasma protein binding
The unbound fractions of S-warfarin and R-warfarin in plasma were assessed by ultrafiltration (Centrifree® YM-30, Millipore, Australia Pty Ltd, North Ryde, NSW, Australia) after rac-warfarin (15 µg) was added to pooled plasma (1 ml) obtained from each subject between 1 and 8, 12–72 and 96–168 h after warfarin dose. This approach relies on the assumption of concentration independent protein binding of warfarin enantiomers, as established by Banfield et al.[19]. The percentage unbound was calculated as the ratio of the concentration of each warfarin enantiomer in the ultrafiltrate to that in plasma.
INR measurement
INR was measured in fresh plasma within 4 h of collection, using a BFT™ II analyser (Dade Behring Diagnostics Pty Ltd, Lane Cove, NSW, Australia) with Thromborel® S reagent (human thromboplastin/calcium reagent for one stage prothrombin time, Dade Behring, Australia).
Platelet aggregation measurement
Platelet aggregation was measured using a whole-blood aggregometer (Chrono-par®, Chrono-log Corporation, Havertown, PA, USA; Edward Keller Australia Pty Ltd, Hallam, Vic, Australia) according to the manufacturer's instructions. Briefly, prewarmed whole blood (1 ml) diluted with normal saline (1 : 2 v/v) was incubated at 37 °C for 2 min. Platelet aggregation was induced by adding arachidonic acid (10 µl, 50 m m stock concentration, Chrono-log Corporation, EKA, Australia). A change in impedance was recorded for 6 min after stimulation with arachidonic acid and reported as impedance aggregation (Ω).
Data treatment
The pharmacokinetic parameters for the warfarin enantiomers were estimated using noncompartmental methods. The elimination rate constant (k) was obtained by linear regression analysis of warfarin enantiomer log concentration-time data over the terminal linear portion of the concentration – time curve. Elimination half-life (t1/2) was calculated as ln2/k. The area under the plasma S-warfarin and R-warfarin concentration-time curves until the last concentration observation (AUC0–t) were calculated using the trapezoidal rule. The AUC was extrapolated to infinity (AUC0–∞) using Ct/k where Ct is the last measured S-warfarin or R-warfarin concentration. The extrapolated portion of AUCt−∞ was less than 5.4% (95% CI, 2.8–8.1%) for S-warfarin and 11.2% (95% CI, 8.9–13.6%) for R-warfarin. The highest S-warfarin and R-warfarin concentrations (Cmax) and the time that these occurred (tmax) were obtained by observation without interpolation. Apparent clearance (CL/F) and apparent volume of distribution (V/F) for the warfarin enantiomers were calculated as Dose/2/AUC0–∞ and CL/F/k, respectively. The area under the INR-time curve until 168 h (AUC0−168 of INR) was calculated by the trapezoidal method. Urinary excretion rate of S-7-hydroxywarfarin was calculated as the amount of metabolite eliminated in urine divided by the sample collection interval.
Statistical analysis
The pharmacokinetic and pharmacodynamic parameters after each treatment were compared by analysis of variance (anova) followed by post hoc multiple comparisons with Dunnett's test using Stata® 5.0 (Stata Corp., Texas, USA) and SPSS® 11.0 (SPSS Inc., Chicago, IL, USA) for subjects nested in sequence, sequence, period and treatment effects. A P-value of less than 0.05 was considered significant. The 95% confidence interval (CI) was used for presentation of study parameters and the 90% CI of the ratio of logarithmically transformed parameters was used to compare control (warfarin alone) and treatment (warfarin with herbal medicine) phases. The significance of the 90% CI of parameter ratios for the primary end points was determined by comparison with the accepted range of 0.80–1.25.
Results
Pharmacokinetics of S-warfarin and R-warfarin
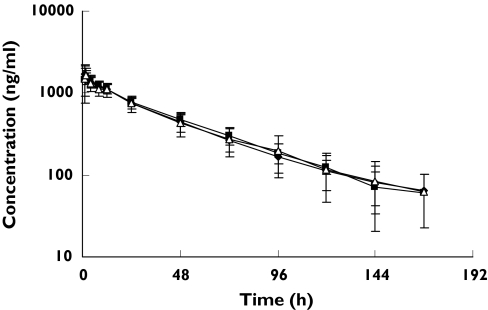
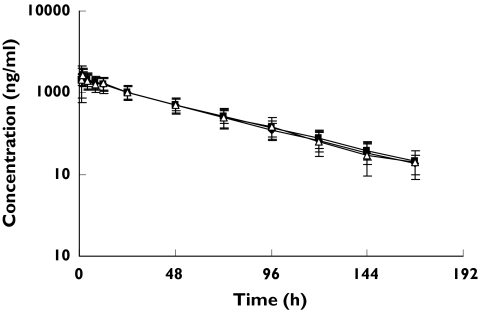
There were no significant changes observed in the pharmacokinetic parameters of S- or R-warfarin in healthy male subjects following treatment with ginkgo or ginger (Figures 1 and 2; Tables 1 and 2).
Figure 1.
(S) warfarin log concentration-time profiles following a single oral 25 mg rac-warfarin dose alone (▪), coadministered with ginkgo (warfarin + ginkgo) (♦), or ginger (▵), (Mean ± SD, n = 12) in healthy male subjects
Figure 2.
(R) warfarin log concentration-time profiles following a single oral 25 mg rac-warfarin dose alone (▪), coadministered with ginkgo (warfarin + ginkgo) (♦), or ginger (▵), (Mean ± SD, n = 12) in healthy male subjects
Table 1.
Warfarin pharmacokinetic parameters following a single oral 25 mg rac-warfarin dose alone or in combination with ginkgo (warfarin + ginkgo) or ginger (warfarin + ginger) in healthy subjects (Mean and 95% CI, n = 12)
| Treatment | Warfarin alone | Warfarin + ginkgo | Warfarin + ginger |
|---|---|---|---|
| Per cent unbound (%) | 0.52 (0.44–0.60) | 0.49 (0.42–0.57) | 0.52 (0.45–0.58) |
| S-warfarin R-warfarin | 0.48 (0.41–0.54) | 0.47 (0.41–0.52) | 0.48 (0.43–0.54) |
| AUC0−8 (µg ml−1 h) | 68.0 (60.8–75.3) | 65.8 (55.1–76.5) | 66.0 (54.0–78.1) |
| S-warfarin R-warfarin | 104.0 (88.4–120.0) | 102.2 (90.8–113.6) | 102.6 (86.4–118.8) |
| tmax (h) S-warfarin | 2.1 (1.4–2.8) | 1.4 (0.9–1.9) | 1.6 (1.1–2.1) |
| R-warfarin | 2.1 (1.4–2.8) | 1.6 (0.6–2.7) | 1.6 (1.1–2.1) |
| Cmax (µg ml−1) S-warfarin | 1.7 (1.4–2.0) | 1.8 (1.5–2.0) | 1.7 (1.5–2.0) |
| R-warfarin | 1.7 (1.4–2.0) | 1.8 (1.5–2.0) | 1.7 (1.5–1.9) |
| t1/2 (h) S-warfarin | 35.8 (31.1–40.3) | 35.1 (30.9–39.3) | 35.7 (30.0–41.3) |
| R-warfarin | 50.3 (45.8–54.9) | 48.6 (44.7–52.4) | 47.7 (42.6–52.8) |
| CL/F (ml h−1) S-warfarin | 189 (167–210) | 200 (173–227) | 201 (171–231) |
| R-warfarin | 127 (106–149) | 126 (111–141) | 131 (106–156) |
| V/F (l kg−1) S-warfarin | 0.12 (0.11–0.14) | 0.12 (0.11–0.13) | 0.12 (0.11–0.13) |
| R-warfarin | 0.12 (0.10–0.13) | 0.11 (0.10–0.12) | 0.11 (0.10–0.12) |
Table 2.
Mean ratios (and 90% confidence intervals) for log transformed S-warfarin and R-warfarin pharmacokinetic parameters comparing pretreatment with an herbal medicine to warfarin alone in healthy subjects (n = 12)
| Treatment | Ginkgo | Ginger |
|---|---|---|
| Per cent unbound S-warfarin R-warfarin | 0.98 (0.90–1.02) 0.99 (0.95–1.05) | 1.02 (0.89–1.15) 1.03 (0.97–1.09) |
| AUC0−∞ S-warfarin R-warfarin | 0.97 (0.89–1.03) 1.00 (0.92–1.07) | 0.95 (0.89–1.03) 0.98 (0.91–1.06) |
| tmax S-warfarin R-warfarin | 0.68 (0.63–0.73) 0.72 (0.67–0.77) | 0.79 (0.73–0.85) 0.79 (0.73–0.85) |
| Cmax S-warfarin R-warfarin | 1.04 (0.97–1.09) 1.03 (0.97–1.10) | 1.01 (0.94–1.07) 1.02 (0.95–1.07) |
| t1/2 S-warfarin R-warfarin | 0.98 (0.93–1.04) 0.97 (0.92–1.02) | 0.99 (0.94–1.04) 0.94 (0.90–1.01) |
| CL/F S-warfarin R-warfarin | 1.05 (0.98–1.12) 1.00 (0.93–1.08) | 1.05 (0.97–1.13) 1.02 (0.95–1.10) |
| V/F S-warfarin R-warfarin | 1.03 (0.99–1.07) 0.98 (0.95–1.01) | 1.03 (0.99–1.08) 0.97 (0.93–1.00) |
Urinary excretion rate of S-7-hydroxywarfarin
The urinary excretion rate of S-7-hydroxywarfarin after administration of warfarin alone was 35 (26–42) µg h−1 and there was no significant difference following treatment with either ginkgo or ginger with urinary excretion rates of 35 (26–44) µg h−1 and 33 (24–41) µg h−1, respectively. The mean ratio (and 90% CI) for S-7-hydroxywarfarin urinary excretion rate was 1.07 (0.85–1.32) and 1.00 (0.81–1.23) following treatment with ginkgo and ginger, respectively.
Protein binding
The mean (95% CI) percentage unbound of S-warfarin and R-warfarin were 0.52% (0.44–0.60%) and 0.48% (0.41–0.54%), respectively, for warfarin alone, and 0.49% (0.42–0.57%) and 0.47% (0.41–0.52%) for respective warfarin enantiomers following administration with ginkgo. Similarly, following treatment with ginger, the percentage unbound of S- and R-warfarin were 0.52% (0.45–0.58%) and 0.48% (0.43–0.54%), respectively. These differences were not significant. Furthermore, protein binding did not change over the time course of sampling (Tables 1 and 2).
Pharmacodynamics
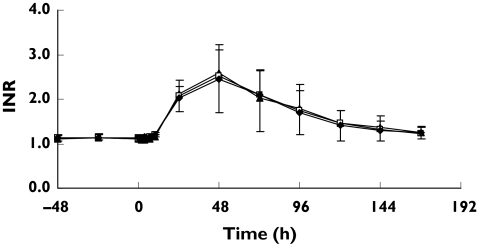
The mean ratios of the AUC0−168 of INR (and 90% CI) were 0.93 (0.82–1.05) following treatment with ginkgo and 1.03 (0.90–1.15) for ginger treatment (Table 4). These data suggest there is no significant effect of these herbs on warfarin pharmacodynamics. Neither ginkgo nor ginger alone affected baseline INR or ex vivo platelet aggregation in response to arachidonic acid (Figure 3, Tables 3 and 4).
Table 4.
Mean ratios (and 90% confidence intervals) for log transformed S-warfarin and R-warfarin pharmacodynamic parameters comparing pretreatment with an herbal medicine to warfarin alone in healthy subjects (n = 12)
| Treatment | Ginkgo (90% CI) | Ginger (90% CI) |
|---|---|---|
| INRbaseline | 1.03 (1.00–1.05) | 1.00 (0.98–1.02) |
| AUC0−168 of INR | 0.93 (0.81–1.05) | 1.01 (0.93–1.15) |
| Platelet aggregation | 1.14 (1.08–1.20) | 1.11 (1.04–1.16) |
Figure 3.
INR-time profiles following a single oral 25 mg rac-warfarin dose alone (□), combination with ginkgo (warfarin + ginkgo) (▴) or ginger (warfarin + ginger) (♦) (Mean ± SD, n = 12, warfarin dose administered at time 0)
Table 3.
Warfarin pharmacodynamic parameters following a single oral 25 mg rac-warfarin dose alone or in combination with ginkgo (warfarin + ginkgo) or ginger (warfarin + ginger) in healthy subjects (Mean and 95% CI, n = 12)
| Treatment | Warfarin alone | Warfarin + ginkgo | Warfarin + ginger |
|---|---|---|---|
| INRbaseline | 1.12 (1.10–1.17) | 1.14 (1.10–1.18) | 1.12 (1.10–1.16) |
| AUC0−168 of INR | 124 (90–158) | 121 (77–165) | 125 (91–160) |
| Platelet aggregation (Ω) | 7.5 (6.5–8.4) | 8.4 (7.6–9.2) | 8.1 (7.5–8.7) |
Adverse events
Twelve subjects completed the study. No significant adverse events were observed. One subject reported gastrointestinal side-effects including constipation during the first 2 days of ginkgo pretreatment and mild diarrhoea during the first 2 days of ginger pretreatment.
Discussion
Co-administration of recommended doses of two commonly used herbal medicines, ginkgo and ginger, did not affect the pharmacokinetics nor the pharmacodynamics of warfarin enantiomers after a single dose of warfarin in healthy male subjects. Furthermore, these herbal medicines did not have an independent effect on blood clotting status or platelet aggregation.
The herbal medicine products of known quality were selected for use in this study and the doses in which they were administered conformed to the recommendations in the Herbal Medicine-Expanded Commission E Monographs. In evaluating the composition of different commercial herbal medicine products we found that products containing ginkgo were qualitatively similar, however, there was notable variability in the composition of the different products containing ginger. This observation reinforces the need to establish the quality of herbal medicine products used in clinical studies. The ginkgo product chosen for the present study (Tavonin™) contained the standardized Ginkgo biloba extract, EGb 761 which has been investigated in numerous clinical trials and has been the subject of a Cochrane Database systematic review [2]. However, it cannot necessarily be assumed that similar findings would result from similar studies of other commercial sources of these extracts. This represents one of the difficulties in studying and interpreting the results of this complex area.
The anticoagulant activity of S-warfarin is several-fold greater than that of the R-enantiomer [20]. Elucidation of the pharmacokinetics of the individual warfarin enantiomers allows an assessment of the possible mechanisms involved in herb–drug interactions. S-warfarin is predominantly metabolized to S-7-hydroxywarfarin by CYP2C9 [21, 22] while R-warfarin is partly metabolized to 6- and 8-hydroxywarfarin by CYP1A2 and to 10-hydroxywarfarin by CYP3A4 [23, 24]. The baseline pharmacokinetic parameters of the enantiomers of warfarin were in agreement with those reported previously [17, 25].
This study used a single 25 mg dose and a cross-over design to investigate possible herb–drug interactions. This design has been safely used by many researchers to investigate potential drug–drug interactions with warfarin and with appropriate sampling this approach allows insight into the possible mechanisms of warfarin drug interactions. A single 25 mg dose of warfarin was used because it induces a significant change in INR from baseline (i.e. maximum INR was approximately 2.5) which allows assessment of the possible additive or reductive effects of coadministration of herbs on warfarin's response. One possibility is that a large dose of warfarin would mean that the maximum pharmacodynamic response is achieved and that amplification of warfarin-induced anticoagulation might not be detected. However, Chan et al.[20] used a combined pharmacokinetic–pharmacodynamic (PK/PD) modelling approach to show that this study design could detect interactions that both increased and decreased INR from the control. Furthermore, in a preliminary analysis using the modelling approach described by Chan et al.[20] we found that only about 80% of the maximum effect of warfarin anticoagulant response was achieved after a single 25 mg dose of warfarin which suggests the study design was sensitive enough to detect both an increase and decrease in INR. This consideration is important given that this study did not detect a significant change in warfarin response when coadministered with either gingko or garlic.
While no interaction was observed between warfarin and ginkgo in this study, ginkgo constituents have been reported to both inhibit and, paradoxically, induce cytochrome P450, depending on the study design [4, 5, 26]. Gurley et al.[27] reported that ginkgo extract (60 mg, 4× day−1 for 28 days) administration to healthy subjects caused no alteration in the activities of CYP3A4, CYP1A2, CYP2E1 or CYP2D6 assessed using a cocktail of specific substrates for individual cytochromes (note: CYP2C9 activity was not investigated). Recently, the effect of ginkgo coadministration on warfarin pharmacodynamics (INR) was investigated using a randomized, double-blind placebo-controlled cross-over trial in patients taking 100 mg of ginkgo extract daily for 4 weeks. These researchers concluded that ginkgo did not influence warfarin response at this dose [28], but the effect of ginkgo on the pharmacokinetics of warfarin enantiomers was not investigated. In the present study, recommended doses of ginkgo did not affect the apparent clearance of warfarin enantiomers, which suggests that this herb (at this dose) does not significantly influence CYP1A2, CYP3A4 and CYP2C9 activity. The finding that S-7-hydroxywarfarin urinary excretion rate was unaltered by ginkgo (and ginger) supports the observation that ginkgo (and ginger) did not affect CYP2C9 activity. The conflicting observations regarding the possible effects of ginkgo constituents on cytochrome P450 could be a consequence of variability in the concentration of different constituents of ginkgo used in different studies. For example, the EGb 761 extract contains less than 5 p.p.m. ginkgolic acid, a constituent known to influence CYP activity in vitro[4, 25].
Several in vitro studies have demonstrated that ginkgo constituents (including ginkgolides) could inhibit platelet activating factor (PAF) but not adenosine diphosphate (ADP) or arachidonic acid induced platelet aggregation [8, 29–31]. Furthermore, collagen induced platelet aggregation was inhibited after an infusion of a ginkgo extract to patients with arteriosclerosis [32]. Similarly, ginkgo extract significantly reduced collagen but not PAF-mediated platelet aggregation in healthy subjects, and in type 2 diabetic subjects who ingested 120 mg of a standardized ginkgo extract for 3 months [9]. However, there is also evidence to suggest that ginkgo does not affect ADP or collagen-induced platelet aggregation in vitro in rats [33]. In a prospective double-blind, randomized placebo-controlled study in healthy subjects, ginkgo extract did not alter platelet activity or coagulation using three different doses (120, 240 and 480 mg day−1) for 14 days [6] which is consistent with the finding of the present study in healthy subjects.
Few ginger–drug interactions have been reported in the literature. A series of synthetic gingerols and phenylalkanol analogues were found to inhibit arachidonic acid induced platelet serotonin release and aggregation based on an in vitro study in human blood [11]. Furthermore, ginger extracts have been reported to inhibit platelet aggregation induced by arachidonic acid, epinephrine, ADP or collagen based on in vitro studies [12–15]. However, in vivo, no significant effect on coagulation or on warfarin response was found in the rat following multiple 100 mg kg−1 doses of ginger extract [34]. There is conflicting evidence related to the effect of ginger constituents on human platelets suggesting that recommended doses (less than 5 g) of ginger do not affect platelet aggregation [16, 35, 36]. In the present study, no significant effect was found on platelet aggregation and coagulation in healthy human subjects who received a daily dose of 3.6 g of ginger for 5 days.
It is not surprising that there are conflicting results of the effects of ginger and ginkgo on clotting status, and on the pharmacokinetics and pharmacodynamics of warfarin, and for herbal drug interactions in general. There is a range of uncontrolled variables across different studies (including differences in the amounts and proportions of constituents in the herbal products depending on their source and preparation) as well as recognized known differences (including aspects of the study design, study population, species differences, variable dosing regimens) [37]. Nevertheless, in the present study we have attempted to control some of these variables and reflect ‘common recommended practice’ by using a standard study design and herbal medicine products of known quality at recommended doses.
In summary, neither ginkgo nor ginger administered in herbal medicine products at recommended doses were found to affect the pharmacokinetics or pharmacodynamics of either S-warfarin or R-warfarin in humans, nor did they affect coagulation status. These findings suggest that the coadministration of ginkgo or ginger at recommended doses is unlikely to cause problems in healthy persons which is consistent with recent controlled trials in the literature. The significance of herb–drug interactions in elderly patients receiving warfarin or in patients taking higher than recommended doses or combinations of these herbal medicines has yet to be established.
Acknowledgments
The authors acknowledge the financial support of the Vincent Fairfax Family Foundation and the National Health and Medical Research Council (NHMRC). The clinical support of the nursing staff at the Clinical Trial Centre, St Vincent's Hospital, Sydney is acknowledged.
References
- 1.Vaes LP, Chyka PA. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34:1478–82. doi: 10.1345/aph.10031. [DOI] [PubMed] [Google Scholar]
- 2.Birks J, Grimley EV, Van Dongen M. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. 2002;4:CD003120. doi: 10.1002/14651858.CD003120. [DOI] [PubMed] [Google Scholar]
- 3.McKenna DJ, Jones K, Hughes K. Efficacy, safety, and use of Ginkgo biloba in clinical and preclinical applications. Altern Ther Health Med. 2001;7:70–86. 88–90. [PubMed] [Google Scholar]
- 4.Zou L, Harkey MR, Henderson GL. Effects of herbal components on cDNA-expressed cytochrome P450 enzyme catalytic activity. Life Sci. 2002;71:1579–89. doi: 10.1016/s0024-3205(02)01913-6. [DOI] [PubMed] [Google Scholar]
- 5.Shinozuka K, Umegaki K, Kubota Y, Tanaka N, Mizuno H, Yamauchi J, et al. Feeding of Ginkgo biloba extract (GBE) enhances gene expression of hepatic cytochrome P-450 and attenuates the hypotensive effect of nicardipine in rats. Life Sci. 2002;70:2783–92. doi: 10.1016/s0024-3205(02)01530-8. [DOI] [PubMed] [Google Scholar]
- 6.Bal Dit Sollier C, Caplain H, Drouet L. No alteration in platelet function or coagulation induced by EGb761 in a controlled study. Clin Laboratory Haematol. 2003;25:251–3. doi: 10.1046/j.1365-2257.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- 7.Umegaki K, Saito K, Kubota Y, Sanada H, Yamada K, Shinozuka K. Ginkgo biloba extract markedly induces pentoxyresorufin O-dealkylase activity in rats. Jpn J Pharmacol. 2002;90:345–51. doi: 10.1254/jjp.90.345. [DOI] [PubMed] [Google Scholar]
- 8.Akiba S, Kawauchi T, Oka T, Hashizume T, Sato T. Inhibitory effect of the leaf extract of Ginkgo biloba L. on oxidative stress-induced platelet aggregation. Biochem Mol Biol Int. 1998;46:1243–8. doi: 10.1080/15216549800204812. [DOI] [PubMed] [Google Scholar]
- 9.Kudolo GB, Dorsey S, Blodgett J. Effect of the ingestion of Ginkgo biloba extract on platelet aggregation and urinary prostanoid excretion in healthy and Type 2 diabetic subjects. Thromb Res. 2002;108:151–60. doi: 10.1016/s0049-3848(02)00394-8. [DOI] [PubMed] [Google Scholar]
- 10.Afzal M, Al-Hadidi D, Menon M, Pesek J, Dhami MS. Ginger: an ethnomedical, chemical and pharmacological review. Drug Metabol Drug Interact. 2001;18:159–90. doi: 10.1515/dmdi.2001.18.3-4.159. [DOI] [PubMed] [Google Scholar]
- 11.Koo KL, Ammit AJ, Tran VH, Duke CC, Roufogalis BD. Gingerols and related analogues inhibit arachidonic acid-induced human platelet serotonin release and aggregation. Thromb Res. 2001;103:387–97. doi: 10.1016/s0049-3848(01)00338-3. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava KC. Isolation and effects of some ginger components of platelet aggregation and eicosanoid biosynthesis. Prostaglandins Leukot Med. 1986;25:187–98. doi: 10.1016/0262-1746(86)90065-x. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava KC. Aqueous extracts of onion, garlic and ginger inhibit platelet aggregation and alter arachidonic acid metabolism. Biomed Biochim Acta. 1984;43:S335–46. [PubMed] [Google Scholar]
- 14.Suekawa M, Yuasa K, Isono M, Sone H, Ikeya Y, Sakakibara I, et al. Pharmacological studies on ginger. IV. Effect of (6)-shogaol on the arachidonic cascade. Nippon Yakurigaku Zasshi. 1986;88:263–9. doi: 10.1254/fpj.88.263. [DOI] [PubMed] [Google Scholar]
- 15.Srivas KC. Effects of aqueous extracts of onion, garlic and ginger on platelet aggregation and metabolism of arachidonic acid in the blood vascular system: in vitro study. Prostaglandins Leukot Med. 1984;13:227–35. doi: 10.1016/0262-1746(84)90014-3. [DOI] [PubMed] [Google Scholar]
- 16.Bordia A, Verma SK, Srivastava KC. Effect of ginger (Zingiber officinale Rosc.) and fenugreek (Trigonella foenumgraecum L.) on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids. 1997;56:379–84. doi: 10.1016/s0952-3278(97)90587-1. [DOI] [PubMed] [Google Scholar]
- 17.Jiang X, Williams KM, Liauw WS, Ammit AJ, Roufogalis BD, Duke CC, et al. Effect of St John's wort and ginseng on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2004;57:592–9. doi: 10.1111/j.1365-2125.2003.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naidong W, Lee JW. Development and validation of a high-performance liquid chromatographic method for the quantitation of warfarin enantiomers in human plasma. J Pharm Biomed Anal. 1993;11:785–92. doi: 10.1016/0731-7085(93)80070-h. [DOI] [PubMed] [Google Scholar]
- 19.Banfield C, O'Reilly R, Chan E, Rowland M. Phenylbutazone–warfarin interaction in man: further stereochemical and metabolic considerations. Br J Clin Pharmacol. 1983;16:669–75. doi: 10.1111/j.1365-2125.1983.tb02239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan E, McLachlan A, O'Reilly R, Rowland M. Stereochemical aspects of warfarin drug interactions: use of a combined pharmacokinetic-pharmacodynamic model. Clin Pharmacol Ther. 1994;56:286–94. doi: 10.1038/clpt.1994.139. [DOI] [PubMed] [Google Scholar]
- 21.Kim JS, Nafziger AN, Gaedigk A, Dickmann LJ, Rettie AE, Bertino JS., Jr Effects of oral vitamin K on S- and R-warfarin pharmacokinetics and pharmacodynamics: enhanced safety of warfarin as a CYP2C9 probe. J Clin Pharmacol. 2001;41:715–22. doi: 10.1177/00912700122010618. [DOI] [PubMed] [Google Scholar]
- 22.Black DJ, Kunze KL, Wienkers LC, Gidal BE, Seaton TL, McDonnell ND, et al. Warfarin-fluconazole. II. A metabolically based drug interaction: in vivo studies. Drug Metab Dispos. 1996;24:422–8. [PubMed] [Google Scholar]
- 23.Chan E, McLachlan AJ, Pegg M, MacKay AD, Cole RB, Rowland M. Disposition of warfarin enantiomers and metabolites in patients during multiple dosing with rac-warfarin. Br J Clin Pharmacol. 1994;37:563–9. doi: 10.1111/j.1365-2125.1994.tb04305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaminsky LS, Zhang ZY. Human P450 metabolism of warfarin. Pharmacol Ther. 1997;73:67–74. doi: 10.1016/s0163-7258(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 25.Breckenridge A, Orme M, Wesseling H, Lewis RJ, Gibbons R. Pharmacokinetics and pharmacodynamics of the enantiomers of warfarin in man. Clin Pharmacol Ther. 1974;15:424–30. doi: 10.1002/cpt1974154424. [DOI] [PubMed] [Google Scholar]
- 26.Ohnishi N, Kusuhara M, Yoshioka M, Kuroda K, Soga A, Nishikawa F, et al. Studies on interactions between functional foods or dietary supplements and medicines. I. Effects of Ginkgo biloba leaf extract on the pharmacokinetics of diltiazem in rats. Biol Pharm Bull. 2003;26:1315–20. doi: 10.1248/bpb.26.1315. [DOI] [PubMed] [Google Scholar]
- 27.Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Cui Y, et al. Cytochrome P450 phenotypic ratios for predicting herb–drug interactions in humans. Clin Pharmacol Ther. 2002;72:276–87. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- 28.Engelsen J, Nielsen JD, Hansen KF. Effect of Coenzyme Q10 and Ginkgo biloba on warfarin dosage in patients on long-term warfarin treatment. A randomized, double-blind, placebo-controlled cross-over trial. Ugeskr Laeger. 2003;165:1868–71. [PubMed] [Google Scholar]
- 29.Steinke B, Muller B, Wagner H. Biological standardization of Ginkgo extracts. Planta Med. 1993;59:155–60. doi: 10.1055/s-2006-959633. [DOI] [PubMed] [Google Scholar]
- 30.Lamant V, Mauco G, Braquet P, Chap H, Douste-Blazy L. Inhibition of the metabolism of platelet activating factor (PAF-acether) by three specific antagonists from Ginkgo biloba. Biochem Pharmacol. 1987;36:2749–52. doi: 10.1016/0006-2952(87)90259-0. [DOI] [PubMed] [Google Scholar]
- 31.Nunez D, Chignard M, Korth R, Le Couedic JP, Norel X, Spinnewyn B, et al. Specific inhibition of PAF-acether-induced platelet activation by BN 52021 and comparison with the PAF-acether inhibitors kadsurenone and CV 3988. Eur J Pharmacol. 1986;123:197–205. doi: 10.1016/0014-2999(86)90660-6. [DOI] [PubMed] [Google Scholar]
- 32.Koltringer P, Eber O. Collagen-induced thrombocyte aggregation in parenteral therapy using Ginkgo biloba. Wien Med Wochenschr. 1989;139:92–4. [PubMed] [Google Scholar]
- 33.Umegaki K, Shinozuka K, Watarai K, Takenaka H, Yoshimura M, Daohua P, et al. Ginkgo biloba extract attenuates the development of hypertension in deoxycorticosterone acetate-salt hypertensive rats. Clin Exp Pharmacol Physiol. 2000;27:277–82. doi: 10.1046/j.1440-1681.2000.03236.x. [DOI] [PubMed] [Google Scholar]
- 34.Weidner MS, Sigwart K. The safety of a ginger extract in the rat. J Ethnopharmacol. 2000;73:513–20. doi: 10.1016/s0378-8741(00)00340-8. [DOI] [PubMed] [Google Scholar]
- 35.Lumb AB. Effect of dried ginger on human platelet function. Thromb Haemost. 1994;71:110–1. [PubMed] [Google Scholar]
- 36.Verma SK, Singh J, Khamesra R, Bordia A. Effect of ginger on platelet aggregation in man. Indian J Med Res. 1993;98:240–2. [PubMed] [Google Scholar]
- 37.Coxeter PD, McLachlan AJ, Duke CC, Roufogalis BD. Herb–drug interactions: an evidence based approach. Curr Med Chem. 2004;11:1513–25. doi: 10.2174/0929867043365198. [DOI] [PubMed] [Google Scholar]