Abstract
Aim
To assess the value of using dexamethasone as an in vivo probe for predicting vinorelbine clearance (CL).
Methods
A population approach (implemented with NONMEM) was used to analyse blood vinorelbine pharmacokinetic data from 20 patients who received a 20-min intravenous infusion of vinorelbine (from 20 to 30 mg m−2). Selected patient clinical data as well as known functional single CYP3A5 and ABCB1 genotype were also tested as covariates.
Results
The best covariate model (with ±95% confidence intervals) was based on dexamethasone plasma clearance (DPC) and alkaline phosphatase (ALP): vinorelbine blood CL (l h−1) = 39.8(± 4.0) × (DPC/13.2)0.524(±0.322) × (ALP/137)−0.198(±0.158). Interindividual variability in vinorelbine CL decreased from 29.7% (model without covariate) to 14.7% when including DPC and ALP. Vinorelbine CL was not correlated with body surface area (BSA) or associated with CYP3A5 and ABCB1 genotype.
Conclusions
These results indicate that individualization of vinorelbine dose would be improved by using dexamethasone clearance rather than BSA. Dexamethasone merits further evaluation as a probe of CYP3A metabolism.
Keywords: cytochrome P450 3A4/ 5, dexamethasone, P-glycoprotein, population pharmacokinetics, vinorelbine
Introduction
Vinorelbine (nor-5′-anhydrovinblastine) is an anticancer drug that belongs to the catharanthus alkaloid family. It inhibits the polymerization of the tubulin monomer, causing disorganization in the dynamics of mitotic spindle microtubules leading to apoptosis [1, 2]. Vinorelbine (Navelbine”) is approved for the treatment of metastatic breast cancer and nonsmall cell lung cancer. It has also demonstrated substantial activity in several other malignant diseases such as refractory multiple myeloma [3], Hodgkin's disease and nonHodgkin's lymphomas. Vinorelbine is eliminated mainly by hepatic CYP3A [4], followed by biliary excretion of metabolites, which results in more than 60% of an i.v. dose being found in the faeces [5(]. The pharmacokinetics of vinorelbine show a large interpatient variability [5], which is consistent with the interindividual variability in CYP3A activity [6, 7].
Close relationships between the area under the blood vinorelbine concentration vs. time curve (AUC) and vinorelbine-induced neutropenia have been reported [8]. A relationship between dose and response rate has also been observed [9].
At present, individual doses are based on body surface area, although vinorelbine clearance is poorly correlated with this parameter [10]. A reduction in dose is recommended during hepatic failure [11]. Indeed, the vinorelbine clearance is decreased in patients with large hepatic metastases [12], or with elevated hepatic markers (transaminases [7, 13], bilirubinaemia [12], alkaline phosphatases [10]). However, no accurate criteria have been proposed to define hepatic failure.
Recently, we have shown that dexamethasone may be used as an index of docetaxel clearance [14]. One-third of the interindividual variability in docetaxel clearance is explained by a combination of dexamethasone plasma clearance, α1-acid glycoprotein serum concentrations, and hepatic metastasis status. Gentile et al.[15] showed in vitro that dexamethasone is mainly metabolized by CYP3A, and that its rate of metabolism is correlated with CYP3A4 expression. These authors concluded that the relatively simple metabolic profile of this synthetic glucocorticoid compared with that of other steroids suggests that it might be a useful in vivo probe of CYP3A4 activity in humans. Moreover, dexamethasone is a substrate of P-glycoprotein (P-gp or MDR1 protein or ABCB1 transporter) [16] as is vinorelbine [17].
The main goal of this study was to assess the use of dexamethasone as an in vivo index of vinorelbine clearance. The effect of genetic polymorphisms of P-gp and CYP3A5 on vinorelbine clearance was also assessed.
Patients and methods
Patients
Only adult patients with histologically or cytologically proven solid malignancies for which vinorelbine was indicated were eligible for this study. Exclusion criteria were contraindications to glucocorticoid use, pregnancy, and the inability to obtain blood. The study was approved by the regional ethics committee: ‘Comité Consultatif pour la Protection des Personnes se prêtant à la Recherche Biomédicale Toulouse I’. Written informed consent was obtained from all patients before they began the study.
Drug administration
Vinorelbine (Navelbine” Pierre Fabre Oncologie, Boulogne, France) was diluted in 50 ml 0.9% saline solution and administered intravenously (i.v.) over 20 min using a pump, at doses ranging from 20 to 30 mg m−2. Patients were treated with vinorelbine as monotherapy or in combination with the 5-fluorouracil. In the latter case, vinorelbine was administered before the 5-fluorouracil infusion.
Each patient received dexamethasone (20 mg Qualimed” diluted in 10 ml 0.9% saline administered i.v. over 5 min) the day before the vinorelbine infusion.
The following prophylactic antiemetic regimens were administered: (i) orally granisetron (2 mg Kytril”), or oral ondansetron (16 mg, Zophren”) 1 h before the vinorelbine infusion in combination or not with methylprednisolone i.v. (1 mg kg−1, Solumedrol”) 30 min before the vinorelbine infusion; (ii) metoclopramide i.v. (1 mg kg−1, Primperan”) and methylprednisolone i.v. (1 mg kg−1, Solumedrol”) both 30 min before the vinorelbine infusion.
Blood and urine sampling
The pharmacokinetics of vinorelbine and dexamethasone were studied only at the first cycle of chemotherapy. Blood samples were collected into heparinized tubes through an in-dwelling cannula at the following times: (i) before infusion, 30 min, 2, and 4 h after the beginning of dexamethasone administration; and (ii) before infusion, at the end of the infusion, and 2, 7 and 24 h after the end of the vinorelbine infusion. Blood samples containing vinorelbine were immediately frozen at −20°C until analyses, whereas those containing dexamethasone were immediately centrifuged at 1760 g for 10 min. The plasma was then removed and stored at −20°C until analyses. After dexamethasone administration, urine was collected for 24 h, divided onto three periods: 0–4 h, 4–8 h, and 8–24 h.
Drug analysis
Analytical grade dexamethasone was obtained from Sigma (Saint-Quentin Fallavier, France). 6-Beta-hydroxydexamethasone (6β-OHD) was prepared from dexamethasone 21-acetate (Steraloids Inc., Newport, Rhode Island, USA) by oxidation with selenium dioxide [18]. This reaction is not stereospecific since both 6β-OHD and 6α-OHD were obtained in a ratio of 85 : 15. Tedious purifications were required to obtain pure 6β-OHD and in a low yield (5%). Optimization of the synthesis and the isolation of 6β-OHD will be published elsewhere. Dexamethasone and 6β-OHD were measured by reverse-phase HPLC with UV detection. Drugs were extracted from both urine and plasma (1.0 ml) by solid-liquid extraction using Waters Oasis” HLB 1-cm3 cartridges (Waters, Milford, MA, USA), which were eluted with 2.0 ml of ethyl acetate (after washing successively with methanol/2% acetic acid 20/80, and methanol/2% ammonium hydroxide 20/80). The eluant was washed with 1% aqueous acetic acid and then with 0.3% (w/v) aqueous sodium hydroxide followed by evaporation to dryness. The residue was dissolved in 130 µl of 50/50 methanol/water, and 100 µl was injected onto a Prontosil 120-5-C18-H (5.0 µm) column (Bischoff, Leonberg, Germany). The mobile phase (delivered at a flow rate of 1 ml min−1) was methanol/water in the proportions 30/70 for the first 12 min, followed by 50/50 for 38 min. Flumethasone (purchased from Sigma) was added before extraction as the internal standard. The detection wavelength was 244 nm. The limits of quantification were 50 ng ml−1 for plasma and urinary concentrations for both dexamethasone and 6β-OHD. The between-day coefficients of variation (CV) were less than 7.0%, 17.0%, 16.1%, and 6.9% for plasma dexamethasone, urinary dexamethasone, plasma 6-β-hydroxydexamethasone, and urinary 6-β-hydroxydexamethasone, respectively.
Vinorelbine blood concentrations were determined by reverse-phase HPLC with UV detection after liquid/liquid extraction [19]. The limit of quantification was 2.5 ng ml−1, the between-day and within-day precision were less than 8.6% and 10.0%, respectively.
Genotyping
CYP3A5 Genotyping of the CYP3A5 A6986G SNP was performed as described previously by Kuehl et al.[20].
MDR1 The MDR1 C3435T, G2677T and G2677A polymorphisms were detected using polymerase chain reaction-based restriction enzyme digestion by Sau3AI, BanI, and BsrI, respectively, as described by Cascorbi et al.[21].
Pharmacokinetic analysis
Concentration vs. time profiles of both vinorelbine and dexamethasone were analysed using a population pharmacokinetic approach implemented on the NONMEM program [22] (version V, level 1.1), which was run with a Compaq Visual Fortran 6.6 compiler.
Dexamethasone
Plasma concentrations of dexamethasone were analysed using the first-order conditional estimation (FOCE) method in order to generate POSTHOC individual plasma dexamethasone clearances.
Vinorelbine
First, individual blood vinorelbine cleatances were determined by an empirical Bayesian estimate using the POSTHOC option according to the method of Nguyen et al.[13]. This method was developed to determine blood vinorelbine clearance accurately from sparse sampling and prior information provided by the analysis of data from 64 patients. A three-compartment model with first order elimination from the central compartment was used. Mean (and CV for interindividual variability) pharmacokinetic parameters values were: CL (blood clearance) = 39.4 l h−1 (30%), Vc (central volume of distribution) = 20.9 l (31%), α (distribution slope) = 0.218 h−1 (19%), V (terminal volume of distribution) = 2230 l (30%), k21 (transfer rate constant from the 2nd to the central compartment) = 0.434 h−1 (0%) and k31 (transfer rate constant from the 3rd to the central compartment) = 0.0321 h−1 (0%).
Second, the influence of the following covariates on blood vinorelbine CL was studied: plasma dexamethasone CL (DPC), total urinary amount of 6β-OHD between 0 and 24 h (T6β-OHD expressed as percentage of dose of dexamethasone administered), the ratio between T6β-OHD and unchanged dexamethasone concentrations in urine (MR), dexamethasone plasma half-life (t1/2), body surface area (BSA), body weight, sex, age, WHO performance status, alanine aminotransferase, aspartate aminotransferase, serum α1-acid glycoprotein, albuminaemia, serum creatinine, lymphocyte count, platelet count, bilirubinaemia, γ-glutamyl transpeptidase, alkaline phosphatase, presence (coded as 1) or absence (0) of liver metastases, MDR-1 genotype (coded as 1 for patients who were both homozygous for thymine at 2677 and 3435, and 0 for other genotypes), CYP3A5 genotype (coded as 1 if at least one CYP3A5*1 allele was present, and 0 if this allele was absent).
The influence of each continuous covariate on vinorelbine clearance (CL) was tested according to the following equation, as exemplified by DPC: TVCL = θ1 × (DPC/mean DPC)θ2, where θ1 is the typical population value of CL (TVCL) for a patient with the mean covariate value, and θ2 is the estimated influential factor for the covariate. For noncontinuous covariates, for example sex (coded as 1 for women, 0 for men): TVCL = θ1 × θ2sex, where θ1 is the mean CL for men, and θ2 is the correction factor for CL in women. Full and reduced models (one parameter less) were compared by the χ2 test of differences between their respective objective function values (OBJ). A change in the OBJ of at least 3.84 (P < 0.05, 1 d.o.f.) was required for the addition of a single parameter to the model. An intermediate model was then obtained including all the significant covariates. A stepwise backward elimination procedure was then carried out. At both steps, the interindividual variability estimate was considered with the change in the OBJ, in order to evaluate the influence of each covariate.
A three-compartment model was implemented to perform covariate analysis, with first order elimination from the central compartment (using the subroutine ADVAN 5, TRANS 1 of the NONMEM program). Typical values of vinorelbine blood CL were expressed as dependent on covariates. Inter-individual variability in vinorelbine blood CL and residual variability were determined from our population study, whereas all other pharmacokinetic parameters (V1, k12, k21, k13 and k31) and omega block were fixed according to values published by Variol et al.[7].
Results
Twenty-three patients were recruited, but the pharmacokinetics of both dexamethasone and vinorelbine were studied in only 20 patients. For two, the urine recovery was not complete, and one patient was excluded because he suffered from a major inflammatory syndrome with multiorgan failure 8 h after vinorelbine administration. Patient details are shown in Table 1. Vinorelbine was administered as monochemotherapy (given weekly or every 15 days) to 14 patients, or in combination with 5-fluorouracil to six patients. Doses of vinorelbine ranged between 29 and 60 mg. All patients were being treated for metastatic malignant disease, as first-line (two patients), second-line (13 patients), or third-line therapy or more (five patients).
Table 1. Patient characteristics (n = 20; female/male = 12/8).
| Patient characteristics | Mean (SD) | Range |
|---|---|---|
| Demographics | ||
| Age (years) | 58 (10) | 41–74 |
| Body surface area (m2) | 1.73 (0.19) | 1.37–2.25 |
| Weight (kg) | 65 (13) | 45–106 |
| Laboratory measurements | ||
| Alanine aminotransferase (IU l−1) | 31 (30) | 12–127 |
| Aspartate aminotransferase (IU l−1) | 40 (39) | 9–178 |
| Alkaline phosphatase (IU l−1) | 137 (98) | 40–407 |
| γ-Glutamyl transpeptidase (IU l−1) | 156 (279) | 15–1174 |
| Bilirubinaemia (µm) | 8.7 (3.8) | 4.7–16.6 |
| Serum α1-acid glycoprotein (g l−1) | 1.2 (0.7) | 0.5–3.2 |
| Albuminaemia (g l−1) | 36 (5) | 25–42 |
| Serum creatinine (µm) | 73 (14) | 55–104 |
| Lymphocyte count (µl−1) | 921 (353) | 132–1500 |
| Platelet count (× 103 µl−1) | 283 (88) | 163–493 |
| Disease and treatment | ||
| WHO performance status* (0/1/2) | 7/10/3 | |
| Vinorelbine dose (mg/m2) | 25 (9) | 20–30 |
| Tumour type: breast/oesophagus/CUP†/lung/others | 6/5/3/2/4 | |
| Liver metastasis (yes/no) | 8/12 | |
World Health Organization performance status: 0, fully active; 1, restricted in physically strenuous activity but ambulatory; 2, ambulatory and capable of all selfcare but unable to carry out any work activities.
Carcinoma of unknown primary.
Only two patients were heterozygously carriers of the CYP3A5*1 allele, all others being homozygous for the CYP3A5*3 allele.
No MDR1 G2677A variant was found. With respect to the MDR1 C3435T and G2677T alleles, six and seven patients, respectively, were homozygous wild type, eight and seven were heterozygotes, and six and six patients were homozygotes for the mutant allele. The two mutations showed a strong linkage, since of the 14 patients carrying at least one mutant allele at the C3435T position, 13 also carried a mutant allele at the G2677T position.
Dexamethasone plasma concentration vs. time profiles were well fitted by a one-compartment model with first order elimination. Residual variability was 12.5%, and interindividual variability for dexamethasone CL was 40.9%. The POSTHOC parameters inferred from the population study are shown in Table 2. No association was observed between either CYP3A5 or MDR1 genotypes and dexamethasone pharmacokinetic parameters. Dexamethasone plasma clearance and the metabolic ratio (MR) were only weakly correlated (r = 0.47, P < 0.05).
Table 2. Mean (SD) values of the pharmacokinetic parameters of dexamethasone (n = 20 patients).
| Pharmacokinetic parameters | Mean (SD) | Range | Abbreviation |
|---|---|---|---|
| Plasma data | |||
| Plasma clearance (L/ h) | 13.2 (4.6) | 4.5–22.0 | DPC |
| Plasma half-life (h) | 3.0 (1.1) | 2.0–6.0 | t1/2 |
| Central volume of distribution (L) | 51.7 (8.3) | 38.6–70.0 | |
| Urinary data | |||
| Amount of 6β-OHdexamethasone* | 12.9% (3.8%) | 4.1–17.9% | T6β-OHD |
| Amount of dexamethasone† | 3.6% (1.1%) | 1.5–5.3% | UD |
| Metabolic ratio between T6β-OHD and | 4.02 (1.50) | 0.77–6.20 | MR |
| unchanged dexamethasone | |||
Percentage of the dose of dexamethasone excreted as 6β-hydroxydexamethasone in urine between 0 and 24 h.
Percentage of the dose of dexamethasone excreted unchanged in urine between 0 and 24 h.
Vinorelbine blood CL determined by Bayesian empirical estimation according to the model described by Nguyen et al.[13] ranged from 15.9 to 55.1 l h−1 with a mean value of 40.2 l h−1. Standardized to BSA, these values ranged from 9.2 to 31.5 l h m−2, with a mean value of 23.5 l h m−2.
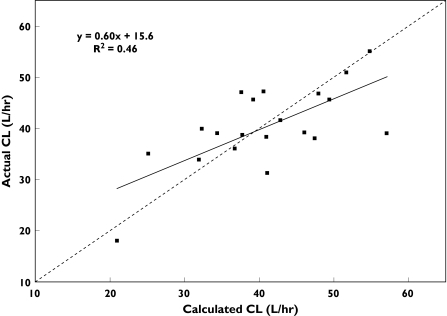
Dexamethasone plasma clearance, plasma half life, the urinary recovery of 6-β-hydroxydexamethasone, and the MR were all significantly correlated with blood vinorelbine CL (Table 3). The relation between blood vinorelbine CL estimated according to the best of the four covariate models [i.e. CL = 41.4 × (DPC/13.2)0.628] and experimentally determined blood vinorelbine CL is shown in Figure 1 (r = 0.68, P < 0.001).
Table 3. Influence of dexamethasone pharmacokinetic parameters on vinorelbine blood clearance and alternative pharmacokinetic models combining dexamethasone plasma clearance and biological markers of hepatic function.
| Models* | Coefficient values (± 95% CI) | ΔOBJ | P | %CV† | R2¶ |
|---|---|---|---|---|---|
| Without covariate | |||||
| CL = θ1 | θ1 = 40.9 (± 5.2) l h−1 | – | – | 29.7 | – |
| ΔOBJ‡ | P | %CV† | |||
| Based on one dexamethasone covariate* | |||||
| CL = θ1 × (DPC/13.2)θ2 | θ1 = 41.4 (± 4.8); θ2 = 0.628 (± 0.426) | − 16.7 | <0.001 | 18.3 | 0.46 |
| CL = θ1 × (MR/4.02)θ2 | θ1 = 41.9 (± 4.4); θ2 = 0.481 (± 0.338) | − 14.9 | <0.001 | 17.7 | 0.39 |
| CL = θ1 × (T6β-OHD/12.9)θ2 | θ1 = 41.2 (± 4.8); θ2 = 0.437 (± 0.570) | − 6.6 | <0.05 | 24.0 | 0.27 |
| CL = θ1 × (T1/2/3.0)θ2 | θ1 = 38.8 (± 4.8); θ2 = −0.679 (± 0.590) | − 10.8 | <0.01 | 20.9 | 0.39 |
| ΔOBJ§ | P | %CV† | |||
| Based on two covariates* | |||||
| CL = θ1 × (DPC/13.2)θ2 × (ALP/137)θ3 | θ1 = 39.8 (± 4.0); θ2 = 0.524 (± 0.322); θ3 = −0.198 (± 0.158) | − 5.2 | <0.025 | 14.7 | 0.57 |
| CL = θ1 × (DPC/13.2)θ2 × (GGT/156)θ3 | θ1 = 38.6 (± 4.4); θ2 = 0.561 (± 0.334); θ3 = −0.078 (± 0.068) | − 4.0 | <0.05 | 16.2 | 0.53 |
| CL = θ1 × (DPC/13.2)θ2 × (MR/4.02)θ3 | θ1 = 41.7 (± 3.6); θ2 = 0.408 (± 0.330); θ3 = 0.298(± 0.294) | − 4.4 | <0.05 | 14.6 | 0.54 |
DPC, Dexamethasone plasma clearance; MR, metabolic ratio; T6β-OHD, percentage of dexamethasone excreted as 6β-hydroxydexamethasone in urine between 0 and 24 h; t1/2, dexamethasone plasma half-life; ALP, alkaline phosphatase; GGT, γ-glutamyl transferase.
Coefficient of variation for interindividual variability not explained by the covariate, if any.
Change in objective function in comparison with the model without covariate.
Change in objective function in comparison with the covariate model based on the DPC alone.
Coefficient of determination between the predicted and actual vinorelbine CL.
Figure 1.
Observed vinorelbine blood clearance vs. values predicted by the covariate model: CL = 41.4 × (DPC/13.2)0.628. DPC, Dexamethasone plasma clearance. The line of identity (- - - -) and the linear regression line (―) are shown
Of the other 18 covariates tested, only ALP and GGT were associated with a significant decrease in the OBJ (−7.5 and −5.1, respectively, P < 0.05 for both covariates). In particular, BSA was not associated with a significant decrease in the OBJ (−0.1, NS), and there was no difference in interindividual variability between the model without a covariate and that incorporating BSA data [i.e. TVCL = 40.9 × (BSA/1.73)−0.161 ± 0.492].
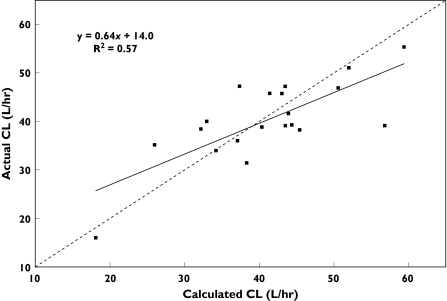
In a second step, combinations of each dexamethasone covariate and relevant biological measurements were tested. The combination of ALP or GGT with DPC (but not with the MR) was associated with a significant decrease in objective function (Table 3. The correlation between blood vinorelbine CL calculated according to the best model based on two covariates [i.e. TVCL = 39.8 × (DPC/13.2)0.524 × (ALP/137)−0.198] and experimentally determined blood vinorelbine CL is shown in Figure 2 (r = 0.75, P < 0.001). An intermediate model based on the three covariates (DPC, ALP, and GGT) was not significantly better than the two covariate model (DPC and ALP). Interindividual variability in CL decreased from 29.7%– free model – to 18.3% incorporating the DPC data alone, and to 14.7% when both the DPC and ALP data were taken into account.
Figure 2.
Observed vinorelbine blood clearance vs. values predicted by the covariate model: CL = 39.8 × (DPC/13.2)0.524 × (ALP/137)−0.198. DPC, Dexamethasone plasma clearance; ALP, alkaline phosphatase. The line of identity (- - - -) and the linear regression line (―) are shown
Discussion
Population pharmacokinetic studies were conducted by the Laboratoire Pierre-Fabre (Boulogne, France) during the clinical development of vinorelbine. Their objectives were to define rational guidelines for the dosing of this anticancer drug based on the correlation between its AUC and the dose-limiting toxicity, neutropenia. The guidelines were derived from results published by Nguyen et al.[13] and Variol et al.[7] The direct relationship between body surface area (BSA) and vinorelbine CL supported dosing based on the former. Trends were detected between CL and some hepatic biochemical markers, but no specific recommendations were made in terms of dose adaptation when values were elevated, since the variability in CL explained by these biochemical markers was low. By performing a population pharmacokinetic study in 30 female patients, Deporte-Fety et al.[10] proposed a dose reduction in patients with increased concentrations of ALP. Robieux et al.[12] have proposed the use of the MEGX (monoethylglycinexylide) liver function test (based on lignocaine metabolite formation) to predict vinorelbine clearance. An excellent correlation (R2 = 0.7) was found between plasma MEGX concentration 45 min after lignocaine i.v. administration (1 mg kg−1) and vinorelbine CL within a group of patients with liver metastases, but the results were less consistent when patients without liver involvement were studied.
Since we had previously observed that dexamethasone may be a valuable index of docetaxel clearance, we decided to evaluate prospectively its use as a probe for the pharmacokinetics of vinorelbine, another anticancer drug that is also a substrate of CYP3A. The use of glucocorticoids prior to vinorelbine administration is not as widespread as it is for docetaxel. However, dexamethasone treatment is more common in cancer patients than the administration of the ‘classical’ probes of CYP3A activity (e.g. midazolam, erythromycin, nifedipine, lidocaine [23]). Furthermore, a dexamethasone test would not require a significant change to patient treatment.
As shown in our previous study, DPC and the MR were significant determinants of vinorelbine CL. The model based on both DPC and the MR was significantly better than that based on each one alone. These results are consistent with the observation that DPC and the MR were only weakly correlated. 6β-OHD is the major metabolite formed by the 6-hydroxylation of dexamethasone, which is catalysed by CYP3A4/5 [15], but other factors also influence the total plasma clearance of dexamethasone. DPC was a better covariate than either the MR or the recovery of T6β-OHD. The additional benefit of this finding is that dexamethasone is more readily available than 6β-OHD.
As shown previously for ALP, elevated hepatic plasma enzymes were also associated with a lower vinorelbine CL. The drug is eliminated mainly by liver metabolism with a relatively high clearance. The best model describing its pharmacokinetics was that combining DPC and ALP. About half of the interindividual variability in vinorelbine CL was explained by these two covariates. In the 20 patients studied, interindividual variability in CL was 29.7% when no covariate was included, a value similar to previous data from a larger population, and decreased to 14.7% with the inclusion of both DPC and ALP (Table 3). By contrast, vinorelbine CL did not correlate with BSA. These results indicate that individualization of vinorelbine dose would be improved by using dexamethasone clearance rather than BSA. However, we should emphasize that the number of patients in our study was limited, and as a result individual values had a strong influence on the relationships tested. For example, when considering Figure 2, the correlation ‘benefited’ from one patient with an experimentally determined CL of 16.0 l h−1 and a value calculated using DPC and ALP of 21.0 l h−1, and was weakened by a patient with values of 39.0 and 57.1 l h−1, respectively. A multicentre study in patients with elevated hepatic enzymes is now planned to evaluate prospectively the present covariate model. If the correlation between vinorelbine blood CL and both DPC and ALP is confirmed, the dexamethasone test could be a useful aid for optimizing vinorelbine dosing, particularly in those patients for whom rational guidelines are not available.
The drug efflux transporter P-glycoprotein (P-gp), expressed in multiple drug resistance phenotypes associated with some cancer cells, is also involved in drug disposition [24]. Thus, P-gp expressed in normal tissues such as the canalicular domain of hepatocytes, the kidney proximal tubule, or the brush border of small intestinal cells, can play an important role in the excretion of drugs from cells. Since vinorelbine is a P-gp substrate, it was hypothesized that a change in P-gp activity might lead to a decreased excretion of the drug, as seen for vinblastine in Mdr1a-/- mice [25]. Hoffmeyer et al.[26] found a correlation between the single nucleotide polymorphism C3435T in the MDR1 gene (that codes for P-gp) and altered P-gp activity, leading to modified digoxin disposition. This polymorphism is highly linked to a nonsynonymous variant G2677T/A, which is also associated with altered transporter function or expression [27]. Since the initial work of Hoffmeyer et al., the influence of genetic MDR1 polymorphisms on the disposition of various P-gp substrates (e.g. digoxin, cyclosporin, fexofenadine, tacrolimus, docetaxel) has been extensively studied. The effects vary with the different drugs, and in some cases, data are conflicting for a particular drug (e.g. digoxin) [28]. We did not find a significant effect of these two polymorphisms on vinorelbine blood CL. Since biliary excretion of unchanged vinorelbine accounts for about 25% of the overall elimination [5], the consequence of decreased biliary excretion (due to altered P-gp function) could be masked by variability in metabolism. Recently, no association was observed between the same variants in the MDR1 gene and vincristine pharmacokinetics [29]. However, genetic variability in P-gp activity may be better explained by considering the haplotype rather than specific polymorphisms [30]. Thus, the lack of correlation between the G2677T and C3435T variants and vinorelbine blood CL does not necessarily indicate that altered P-gp function has no influence on vinorelbine pharmacokinetics.
The substantial interindividual variability in constitutive CYP3A activity could be explained by genetic polymorphisms in the CYP3A4 and/or CYP3A5 genes (since total CYP3A activity is the sum of the activities of these isoforms). None of the known allelic variants of CYP3A4 account for variability in CYP3A activity [31]. On the other hand, the CYP3A5 A6986G (CYP3A5*3) variant described by Kuehl et al.[19] has been proposed as the basis of the variability in CYP3A5 expression in adults. CYP3A5*3 introduces a splice site that produces a truncated and nonfunctional protein, whereas the functional protein is expressed in carriers of the wild-type gene (CYP3A5*1). It is reasonable to postulate that CYP3A activity is greater in individuals carrying at least one CYP3A5*1 allele. However, we did not observe a higher vinorelbine CL in patients possessing CYP3A5*1. Thus, for vinorelbine as well as for other CYP3A substrates such as docetaxel [32], and midazolam [33], the relative contribution of CYP3A5 to overall CYP3A metabolism may be limited.
In conclusion, the present study, along with our previous report of a correlation between dexamethasone CL and docetaxel CL, shows that dexamethasone may be a useful predictor of the clearance of other drugs metabolized by CYP3A.
Acknowledgments
F.P. was supported by a grant from the ‘Association pour la Recherche en Cancérologie’.
References
- 1.Wang LG, Liu XM, Kreis W, Budman DR. The effect of antimicrotubule agents on signal transduction pathways of apoptosis: a review. Cancer Chemother Pharmacol. 1999;44:355–61. doi: 10.1007/s002800050989. [DOI] [PubMed] [Google Scholar]
- 2.Aapro MS, Harper P, Johnson SA, Vermorken JB. Developments in cytotoxic chemotherapy: advances in treatment utilising vinorelbine. Crit Rev Oncol Hematol. 2001;40:251–63. doi: 10.1016/s1040-8428(01)00167-6. [DOI] [PubMed] [Google Scholar]
- 3.Ochiai N, Shimazaki C, Inaba T, Fuchida S, Okano A, Hatsuse M, Hirai H, Ashihara E, Fujita N, Nakagawa M. Effect of vinorelbine on the growth of human myeloma cell lines in vitro. Leuk Res. 2002;26:731–8. doi: 10.1016/s0145-2126(01)00195-3. [DOI] [PubMed] [Google Scholar]
- 4.Kajita J, Kuwabara T, Kobayashi H, Kobayashi S. CYP3A4 is mainly responsibile for the metabolism of a new vinca alkaloid, vinorelbine, in human liver microsomes. Drug Metab Dispos. 2000;28:1121–7. [PubMed] [Google Scholar]
- 5.Leveque D, Jehl F. Clinical pharmacokinetics of vinorelbine. Clin Pharmacokinet. 1996;31:184–97. doi: 10.2165/00003088-199631030-00003. [DOI] [PubMed] [Google Scholar]
- 6.Gauvin A, Pinguet F, Culine S, Astre C, Cupissol D, Bressolle F. Blood and plasma pharmacokinetics of vinorelbine in elderly patients with advanced metastatic cancer. Cancer Chemother Pharmacol. 2002;49:48–56. doi: 10.1007/s00280-001-0378-2. [DOI] [PubMed] [Google Scholar]
- 7.Variol P, Nguyen L, Tranchand B, Puozzo C. A simultaneous oral/intravenous population pharmacokinetic model for vinorelbine. Eur J Clin Pharmacol. 2002;58:467–76. doi: 10.1007/s00228-002-0506-x. [DOI] [PubMed] [Google Scholar]
- 8.Khayat D, Rixe O, Brunet R, Goupil A, Bugat R, Harousseau JL, Ifrah N, Puozzo C. Pharmacokinetic linearity of i.v. vinorelbine from an intra-patient dose escalation study design. Cancer Chemother Pharmacol. 2004;54:193–205. doi: 10.1007/s00280-004-0794-1. [DOI] [PubMed] [Google Scholar]
- 9.Toussaint C, Izzo J, Spielmann M, Merle S, May-Levin F, Armand JP, Lacombe D, Tursz T, Sunderland M, Chabot GG. Phase I/II trial of continuous infusion vinorelbine for advanced breast cancer. J Clin Oncol. 1994;12:2102–12. doi: 10.1200/JCO.1994.12.10.2102. [DOI] [PubMed] [Google Scholar]
- 10.Deporte-Fety R, Simon N, Fumoleau P, Campone M, Kerbrat P, Bonneterre J, Fargeot P, Urien S. Population pharmacokinetics of short intravenous vinorelbine infusions in patients with metastatic breast cancer. Cancer Chemother Pharmacol. 2004;53:233–8. doi: 10.1007/s00280-003-0729-2. [DOI] [PubMed] [Google Scholar]
- 11.Summary of Product Characteristics. Boulogne: Pierre Fabre Médicament; 2000. [Google Scholar]
- 12.Robieux I, Sorio R, Borsatti E, Cannizzaro R, Vitali V, Aita P, Freschi A, Galligioni E, Monfardini S. Pharmacokinetics of vinorelbine in patients with liver metastases. Clin Pharmacol Ther. 1996;59:32–40. doi: 10.1016/S0009-9236(96)90021-1. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen L, Tranchand B, Puozzo C, Variol P. Population pharmacokinetics model and limited sampling strategy for intravenous vinorelbine derived from phase I clinical trials. Br J Clin Pharmacol. 2002;53:459–68. doi: 10.1046/j.1365-2125.2002.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puisset F, Chatelut E, Dalenc F, Busi F, Cresteil T, Azema J, Poublanc M, Hennebelle I, Lafont T, Chevreau C, Roche H. Dexamethasone as a probe for docetaxel clearance. Cancer Chemother Pharmacol. 2004;54:265–72. doi: 10.1007/s00280-004-0823-0. [DOI] [PubMed] [Google Scholar]
- 15.Gentile DM, Tomlinson ES, Maggs JL, Park BK, Back DJ. Dexamethasone metabolism by human liver in vitro. Metabolite identification and inhibition of 6-hydroxylation. J Pharmacol Exp Ther. 1996;277:105–12. [PubMed] [Google Scholar]
- 16.Ueda K, Okamura N, Hirai M, Tanigawara Y, Saeki T, Kioka N, Komano T, Hori R. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J Biol Chem. 1992;267:24248–52. [PubMed] [Google Scholar]
- 17.Adams DJ, Knick VC. P-glycoprotein mediated resistance to 5′-nor-anhydro-vinblastine (Navelbine) Invest New Drugs. 1995;13:13–21. doi: 10.1007/BF02614215. [DOI] [PubMed] [Google Scholar]
- 18.Terasawa T, Okada T. Selenium dioxide oxidation of steroidal 1,4–dien-3-ones. A simple and convenient route to 6-hydroxycorticoids. Synthetic Commun. 1991;21:307–17. [Google Scholar]
- 19.Puozzo C, Ung HL, Zorza G. A new sensitive and reliable HPLC method for clinical routine analysis of vinorelbine and 17-deacetyl-vinorelbine, in blood, plasma and urine. J Chromatogr B Analyt. Technol. Biomed. Life Sci. (in press) [Google Scholar]
- 20.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–91. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 21.Cascorbi I, Gerloff T, Johne A, Meisel C, Hoffmeyer S, Schwab M, Schaeffeler E, Eichelbaum M, Brinkmann U, Roots I. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther. 2001;69:169–74. doi: 10.1067/mcp.2001.114164. [DOI] [PubMed] [Google Scholar]
- 22.Beal SL, Sheiner LB. Estimating population kinetics. Crit Rev Biomed Eng. 1982;8:195–222. [PubMed] [Google Scholar]
- 23.Streetman DS, Bertino JS, Jr, Nafziger AN. Phenotyping of drug-metabolizing enzymes in adults. a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 2000;10:187–216. doi: 10.1097/00008571-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Litman T, Druley TE, Stein WD, Bates SE. From MDR to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol Life Sci. 2001;58:931–59. doi: 10.1007/PL00000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Asperen J, Schinkel AH, Beijnen JH, Nooijen WJ, Borst P, van Tellingen O. Altered pharmacokinetics of vinblastine in Mdr1a P-glycoprotein-deficient mice. J Natl Cancer Inst. 1996;88:994–9. doi: 10.1093/jnci/88.14.994. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, Brinkmann U. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473–8. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurata Y, Ieiri I, Kimura M, Morita T, Irie S, Urae A, Ohdo S, Ohtani H, Sawada Y, Higuchi S, Otsubo K. Role of human MDR1 gene polymorphism in bioavailability and interaction of digoxin, a substrate of P-glycoprotein. Clin Pharmacol Ther. 2002;72:209–19. doi: 10.1067/mcp.2002.126177. [DOI] [PubMed] [Google Scholar]
- 28.Marzolini C, Paus E, Buclin T, Kim RB. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004;75:13–33. doi: 10.1016/j.clpt.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Plasschaert SL, Groninger E, Boezen M, Kema I, de Vries EG, Uges D, Veerman AJ, Kamps WA, Vellenga E, de Graaf SS, de Bont ES. Influence of functional polymorphisms of the MDR1 gene on vincristine pharmacokinetics in childhood acute lymphoblastic leukemia. Clin Pharmacol Ther. 2004;76:220–9. doi: 10.1016/j.clpt.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Sakaeda T, Nakamura T, Okumura K. Pharmacogentics of MDR1 and its impact on the pharmacokinetics and pharmacodynamics of drugs. Pharmacogenomics. 2003;4:397–410. doi: 10.1517/phgs.4.4.397.22747. [DOI] [PubMed] [Google Scholar]
- 31.Lamba JK, Lin YS, Schuetz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2002;54:1271–94. doi: 10.1016/s0169-409x(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 32.Goh BC, Lee SC, Wang LZ, Fan L, Guo JY, Lamba J, Schuetz E, Lim R, Lim HL, Ong AB, Lee HS. Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol. 2002;20:3683–90. doi: 10.1200/JCO.2002.01.025. [DOI] [PubMed] [Google Scholar]
- 33.Shih PS, Huang JD. Pharmacokinetics of midazolam and 1′-hydroxymidazolam in Chinese with different CYP3A5 genotypes. Drug Metab Dispos. 2002;30:1491–6. doi: 10.1124/dmd.30.12.1491. [DOI] [PubMed] [Google Scholar]