Abstract
Aims
Previous studies have demonstrated that the antihistamines mequitazine, cetirizine and dexchlorpheniramine produce mild sedation after single doses. It is unknown, however, whether acute sedation persists after repeated dosing. Therefore, this study assessed the effects of repeated dosing of these antihistamines on driving and psychomotor performance.
Methods
Sixteen healthy volunteers were treated with mequitazine 10 mg q.a.m., cetirizine 10 mg q.a.m., dexchlorpheniramine Repetab 6 mg b.i.d. and placebo for four separate 8-day periods. Drug effects were assessed on days 1 and 8 using on-the-road driving tests (highway driving and car following), psychomotor tests (tracking and divided attention) and subjective questionnaires.
Results
Dexchlorpheniramine and mequitazine significantly impaired driving performance on the highway driving test on the first day; dexchlorpheniramine increased Standard Deviation of Lateral Position by 2 cm [95% confidence interval (CI) 0.5, 3.8] and mequitazine by 2.5 cm (CI 1.0, 4.3). These effects on driving performance disappeared after 8 days of treatment. No effect of treatment was found on car following, tracking and divided attention. Although subjective ratings confirmed that subjects knew their driving had been impaired in the mequitazine and dexchlorpheniramine condition after completion of the highway driving test on day 1, they did not expect their driving to be affected before the start of the test. Cetirizine did not impair performance on any of the tests.
Conclusions
Single doses of mequitazine 10 mg and dexchlorpheniramine Repetab 6 mg cause mild driving impairment. However, when taken over several days, the impairing effect wears off, possibly as a result of tolerance.
Keywords: antihistamine, cetirizine, driving, mequitazine, psychomotor performance, repeated-dose
Introduction
Antihistamines (H1-antagonists) are widely used to treat allergies and do so by blocking peripheral H1-receptors. However, these drugs also cross the blood–brain barrier and block neuronal histamine transmission as well as other neurotransmitter systems (e.g. serotonin, acetylcholine, noradrenaline) in the central nervous system [1]. As a consequence they produce sleepiness, fatigue and sedation [2]. The sedative effects of antihistamines can lead to diminished concentration or productivity and an increased risk for occupational injuries and traffic accidents [3–5]. Therefore it is necessary to study and compare the sedative and potentially impairing effects of all antihistamines.
First-generation antihistamines have been shown to produce significant performance impairment in psychomotor and driving tests [6–8]. More recent antihistamines, such as cetirizine, loratadine, ebastine and terfenadine, were developed to overcome the sedative side-effects of the first-generation antihistamines. These second-generation antihistamines cross the blood–brain barrier less readily, and consequently are less sedating than their predecessors when given in the recommended dose [9]. Experimental studies have shown that single therapeutic doses of second-generation antihistamines produce little or no impairment of attention, memory, vigilance, psychomotor performance or driving [6–8, 10]. Higher doses, however, have occasionally been found to produce significant sedative effects, although less pronounced than those of first-generation antihistamines [11–14].
Since antihistamines are usually taken for periods longer than 1 day in the treatment of allergic rhinitis or urticaria, it is relevant to assess their effects not only after single doses, but also after repeated doses, when blood levels are at steady state. Studies have shown that their impairing effects can either diminish or increase over time [6, 7, 13, 15–21]. On the one hand, the effects of triprolidine (5 and 10 mg), diphenhydramine (50 mg) and clemastine (2 mg) on driving and psychomotor performance were found to diminish, although not disappear with repeated dosing [15–21]. On the other hand, high doses of ebastine (30 mg) and terfenadine (120 mg) were found to produce sedation only after repeated doses [6, 7, 13]. It seems therefore that effects can diminish over time as a result of tolerance, or increase as a result of accumulation, depending on the drug and dose.
The objective of the present study was to asses the effects of repeated doses of mequitazine 10 mg, dexchlorpheniramine Repetab 6 mg and cetirizine 10 mg that were previously shown to produce performance impairment after single doses [22–28]. Impairing effects were assessed using two validated driving tests and psychomotor tests previously shown to be sensitive to antihistamine-induced sedation [21, 26, 29].
Methods
Subjects
Sixteen healthy volunteers (eight males and eight females) aged between 22 and 45 years were recruited by means of newspaper advertisements. Subjects were healthy according to medical history, physical examination, electrocardiogram, blood and urine analysis. All participants possessed a valid driver's license for at least 3 years and had driving experience of at least 5000 km per year. Subjects with a history of major medical or psychiatric disorders were excluded from the study, as were subjects with unusual sensitivity to antihistamines, drug abuse and chronic use of medication (except contraceptives). Women who were not using an approved contraception or women who were pregnant or lactating were excluded. Written informed consent was acquired from each subject prior to participation. The study was approved by the Ethics Committee of Maastricht University and conducted in accordance with the World Medical Association's Declaration of Helsinki (Edinburgh modification, 2000).
One male subject dropped out for reasons unrelated to the treatment. Mean ± SD age of the remaining 15 participants was 32 ± 9.1 years and mean weight ± SD was 73.7 ± 15.1 kg (women 64.5 ± 10.8 kg; men 84.3 ± 12.4 kg). All subjects were nonsmokers and used an average of 2.7 (± 2.2) caffeine-containing beverages per day.
Drug administration
This study was conducted according to a 4-way, double-blind, cross-over design. Treatments consisted of regular therapeutic doses: mequitazine 10 mg once daily in the morning (q.a.m.), cetirizine 10 mg q.a.m.; dexchlorpheniramine 6 mg (Repetab) twice daily (b.i.d.), and placebo (b.i.d.). Treatment duration was 8 days starting on the morning of day 1. Tests were conducted on days 1 and 8 of each treatment period. The washout period between treatment periods was at least 7 days.
On test days, drugs and placebo were administered using a double dummy technique, since cetirizine is more rapidly absorbed than mequitazine and dexchlorpheniramine; cetirizine reaches peak plasma concentrations (Tmax) approximately 1 h after oral dosing, whereas mequitazine and dexchlorpheniramine reach Tmax between 3 and 4 h after administration. Therefore, mequitazine and dexchlorpheniramine were administered at 09.00 h, i.e. 3 h before driving, with a placebo capsule at 11.00 h to maintain blinding. Cetirizine was administered at 11.00 h, i.e. 1 h before driving, with a placebo capsule at 09.00 h to ensure blinding. In the placebo condition, placebo capsules were administered at both times. Between test days, subjects continued treatments at home, taking one capsule in the morning and one in the evening. In the mequitazine and cetirizine conditions, the active drug was taken in the morning while a placebo was taken at night. Dexchlorpheniramine and placebo were both taken in the morning and in the evening in the dexchlorpheniramine and placebo conditions, respectively. Subjects noted the times of drug administration in a diary, as well as the use of any concomitant medication.
Procedure
Subjects were individually trained in all tests in the 2 weeks prior to their first treatment period. Subjects had to abstain from alcohol the day before testing, and agreed to have a good night's rest of at least 7 h. On test days consumption of caffeine was not allowed and smoking was prohibited 30 min before and during testing. On test days, subjects were transported to the laboratory where drugs were administered at 09.00 and 11.00 h. At arrival, sleep quality of the previous night was measured using the Groningen Sleep Quality Scale. At 10.00 h they received a standardized light meal. The highway-driving test started at 12.00 h and the car-following test at 13.00 or 13.30 h. Laboratory tests were started at 14.30 h.
Performance measures
Tests used in this study have been described in more detail in previous papers [18, 25].
Driving tests
In the highway-driving test, the subject drives a specially instrumented vehicle over a 100-km distance on a primary highway. The subject has to maintain a steady lateral position between the delineated boundaries of the right (slower) traffic lane and a constant speed of 95 km/h. The primary dependent measure is the standard deviation of lateral position (SDLP), which is an index of the continuous road tracking error.
In the car-following test subjects drive a specially instrumented car and follow a vehicle controlled by an experimenter. The latter or ‘leading' car occasionally changes speed and briefly lights up its brake lights. Subjects are required to keep driving at the same speed as the leading vehicle and to maintain a 15–30-m distance between the cars. In addition, subjects have to react as quickly as possible to the brake lights of the leading car. The primary dependent measures are time to speed adaptation (in ms), and reaction time (in ms) to the brake lights. Deviation in distance to the leading car (in metres) is a secondary parameter.
During both driving tests a licensed driving instructor accompanies the subject, and can intervene when necessary by using dual controls.
Psychomotor tests
The Critical Tracking Test measures the subjects' ability to control a displayed error signal in a first-order compensatory tracking task. The frequency at which control is lost is called the ‘critical frequency' or ‘lambdac' (rad/s). The test includes five trails; the average score is calculated after removing the highest and the lowest scores, and is the primary variable in this test.
The Divided Attention Task is used to measure the subject's ability to divide attention between two tasks performed simultaneously. The primary task consists of a tracking task in the central visual field and the secondary task is a monitoring task in the peripheral visual field. Subjects react to peripheral targets using a foot pedal. Dependent variables are the absolute mean tracking error over the entire test and mean reaction time to peripheral targets.
Subjective measures
Sleep quality of the previous night was assessed by means of the Groningen Sleep Quality Scale [30]. This questionnaire scores the number of sleep complaints, ranging from 0 (good sleep) to 14 (bad sleep), and specific questions about the total amount of sleep, number of awakenings and the estimated time needed to fall asleep.
Subjective driving questionnaires were used in order to evaluate whether patients' subjective experience of their driving ability corresponds to their objective performance on the driving tests. Prior to the highway driving test, subjects estimated their driving ability and after completion of the test they rated driving performance, using 100-mm visual analogue scales ranging from 0 (very bad driving) to 100 (very good driving).
Statistical analysis
Driving and psychomotor parameters were analysed separately for days 1 and 8, using an anova with Subject, Treatment and Period as factors. Subsequently, analyses for comparing treatments with placebo were conducted, using three simple contrasts with sequential Bonferroni correction. In case of a significant effect, one-tailed, 95% confidence intervals of drug-placebo differences were calculated (CI). The effects of gender on driving performance were analysed, using a model including Treatment, Period, Gender and Gender by Treatment. All statistical tests were conducted using SPSS (version 10.0; SPSS Inc., Chicago, IL, USA).
Results
Missing data
Due to technical problems, the car-following test could not be performed by two subjects on two separate occasions. Distance measures between the cars was lost for another two subjects on two separate occasions, also as a result of technical problems.
Driving performance
Four highway driving tests of a single subject were terminated before scheduled completion, because the driving instructor (on days 1 and 8 of the placebo condition and day 1 of the dexchlorpheniramine condition) or the subject (day 8 of cetirizine treatment) judged it would be unsafe to continue. The respective rides were 91%, 90%, 68% and 50% complete when the test was stopped. SDLP scores calculated over the completed parts of these rides were 23.8, 24.6, 32.7 and 24.6 cm, respectively. Statistical analysis of datasets including or excluding scores of incomplete rides revealed comparable results, therefore incomplete rides were included in the analysis.
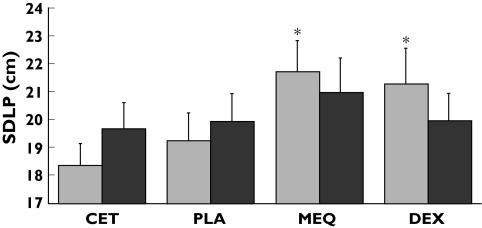
A summary of descriptives and quantitative statistical analyses of all performance parameters is presented in Table 1. Mean (SEM) SDLP in each condition and for both days is shown in Figure 1.
Table 1.
Summary of results from the driving and psychomotor tests on day 1 and day 8
| Mean ± SEM per treatment | Treatment effect | |||||||
|---|---|---|---|---|---|---|---|---|
| CET | PLA | MEQ | DEX | F | d.f. | P | N | |
| Day 1 | ||||||||
| Driving tests | ||||||||
| SDLP (cm) | 18.4 ± 0.8 | 19.2 ± 1.0 | 21.7 ± 1.1a | 21.2 ± 1.3a | 8.16 | 3,39 | <0.001 | 15 |
| Time to speed adaptation (s) | 2.1 ± 0.3 | 1.9 ± 0.2 | 2.3 ± 0.3 | 2.0 ± 0.4 | 1.02 | 3,38 | NS | 14 |
| Brake reaction time (ms) | 537 ± 24 | 476 ± 19 | 528 ± 29 | 492 ± 26 | 2.36 | 3,38 | NS | 14 |
| SD distance (m) | 5.3 ± 0.7 | 5.2 ± 1.1 | 4.3 ± 0.7 | 4.0 ± 0.5 | 0.74 | 3,38 | NS | 15 |
| Critical tracking task | ||||||||
| Critical frequency (rad/s) | 4.0 ± 0.2 | 3.9 ± 0.2 | 3.8 ± 0.2 | 3.9 ± 0.2 | 1.21 | 3,39 | NS | 15 |
| Divided attention task | ||||||||
| Tracking error (mm) | 20.4 ± 1.4 | 21.2 ± 1.5 | 21.1 ± 1.2 | 22.9 ± 1.4 | 2.83 | 3,39 | 0.051 | 15 |
| Reaction time (ms) | 1807 ± 69 | 1747 ± 64 | 1799 ± 54 | 1809 ± 70 | 0.76 | 3,39 | NS | 15 |
| Day 8 | ||||||||
| Driving tests | ||||||||
| SDLP (cm) | 19.6 ± 1.0 | 19.9 ± 1.0 | 21.0 ± 1.2 | 19.9 ± 1.0 | 0.99 | 3,39 | NS | 15 |
| Time to speed adaptation (s) | 2.0 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.3 | 2.0 ± 0.2 | 0.80 | 3,38 | NS | 14 |
| Brake reaction time (ms) | 510 ± 27 | 506 ± 23 | 521 ± 33 | 484 ± 14 | 0.60 | 3,38 | NS | 14 |
| SD distance (m) | 5.1 ± 0.8 | 5.5 ± 1.2 | 5.0 ± 0.7 | 4.5 ± 0.8 | 0.41 | 3,36 | NS | 12 |
| Critical tracking task | ||||||||
| Critical frequency (rad/s) | 3.9 ± 0.2 | 4.0 ± 0.2 | 4.0 ± 0.2 | 4.0 ± 0.2 | 0.32 | 3,39 | NS | 15 |
| Divided attention task | ||||||||
| Tracking error (mm) | 20.4 ± 1.3 | 20.0 ± 1.3 | 20.6 ± 1.3 | 20.9 ± 1.6 | 0.39 | 3,39 | NS | 15 |
| Reaction time (ms) | 1832 ± 69 | 1787 ± 98 | 1692 ± 59 | 1721 ± 77 | 1.53 | 3,39 | NS | 15 |
Treatments are placebo (PLA), mequitazine 10 mg q.a.m. (MEQ), cetirizine 10 mg q.a.m. (CET) and dexchlorpheniramine 6 mg Repetab b.i.d. (DEX). Significant drug–placebo differences are indicated by SDLP, standard deviation of lateral position.
P < 0.017. NS, Not significant; d.f., degrees of freedom.
Figure 1.
Mean (+SEM) Standard Deviation of Lateral Position for each treatment on day 1 ( ) and day 8 (
) and day 8 ( ). Treatments are cetirizine 10 mg q.a.m. (CET), placebo (PLA), mequitazine 10 mg q.a.m. (MEQ), and dexchlorpheniramine 6 mg Repetab twice daily (DEX). Asterisks indicate significant differences from placebo after sequential Bonferroni correction, i.e. P < 0.017
). Treatments are cetirizine 10 mg q.a.m. (CET), placebo (PLA), mequitazine 10 mg q.a.m. (MEQ), and dexchlorpheniramine 6 mg Repetab twice daily (DEX). Asterisks indicate significant differences from placebo after sequential Bonferroni correction, i.e. P < 0.017
The overall effect of Treatment on SDLP was significant on day 1 (F3,39 = 8.16; P < 0.001) but not on day 8. Contrasting each drug with placebo showed that on day 1, SDLP was significantly increased after treatment with mequitazine (mean 2.5; CI 1.03, 4.25; F1,39= 11.03; P = 0.002) and dexchlorpheniramine (mean 2.0; CI 0.46, 3.68; F1,39 = 6.75; P = 0.013), but not after cetirizine (mean 0.8; F1,39 = 0.79). The overall effect of Period on SDLP was significant on days 1 and 8 (F3,39 = 4.15; P = 0.012; F3,39 = 4.69; P = 0.007). Contrasting driving performance in period 1 with performance in subsequent periods revealed no systematic change over time, however, and, more importantly, no interactions were found between Treatment and Period. There were no significant main effects of Gender or Gender by Treatment interactions on days 1 or day 8.
Performance in the car-following test was not significantly affected by Treatments, Periods or Gender on day 1 or day 8. However, it should be mentioned that the incompleteness of the car-following data might be related to the absence of an effect.
Psychomotor tests
A summary of descriptives and statistical analyses of the psychomotor tests is also presented in Table 1. Tracking performance in the critical tracking test was not significantly affected on days 1 or 8, and there was only a nearly significant overall effect of Treatment on tracking in the divided attention task (F1,39 = 2.83; P = 0.051) on the first day of treatment. Subjects achieved the smallest tracking error in the cetirizine condition and the largest in the dexchlorpheniramine condition. However, drug–placebo contrasts showed no significant differences.
A summary of descriptives and quantitative statistical analyses of the subjective measures is presented in Table 2.
Table 2.
Summary of results of the subjective measures on day 1 and day 8
| Mean ± s.e.mean per treatment | Treatment effect | |||||||
|---|---|---|---|---|---|---|---|---|
| CET | PLA | MEQ | DEX | F | d.f. | P | N | |
| Day 1 | ||||||||
| Subjective driving quality | ||||||||
| Pre-test (mm) | 78.5 ± 4.6 | 73.7 ± 6.3 | 79.1 ± 6.5 | 68.7 ± 6.3 | 1.06 | 3,39 | NS | 15 |
| Post-test (mm) | 64.7 ± 6.9 | 67.9 ± 6.6 | 55.1 ± 7.5b | 51.5 ± 8.0a | 4.55 | 3,39 | 0.008 | 15 |
| Sleep questionnaire | ||||||||
| Time before asleep (min) | 18.9 ± 4.6 | 12.4 ± 2.2 | 18.8 ± 4.5 | 22.6 ± 5.0 | 1.06 | 3,39 | NS | 15 |
| Number of awakenings | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.0 ± 0.3 | 1.1 ± 0.4 | 0.00 | 3,39 | NS | 15 |
| Sleep (h) | 6.7 ± 0.3 | 7.6 ± 0.2 | 7.2 ± 0.2 | 7.1 ± 0.3 | 2.05 | 3,39 | NS | 15 |
| Number of complaints | 2.6 ± 0.9 | 1.0 ± 0.4 | 1.7 ± 0.9 | 2.7 ± 0.9 | 1.23 | 3,39 | NS | 15 |
| Day 8 | ||||||||
| Subjective driving quality | ||||||||
| Pre-test (mm) | 86.7 ± 3.0 | 77.5 ± 5.8 | 73.0 ± 4.2 | 72.2 ± 6.4 | 2.66 | 3,39 | NS | 15 |
| Post-test (mm) | 62.7 ± 7.8 | 64.3 ± 5.7 | 59.5 ± 6.5 | 62.3 ± 6.5 | 0.23 | 3,39 | NS | 15 |
| Sleep questionnaire | ||||||||
| Time before asleep (min) | 22.9 ± 7.1 | 22.3 ± 6.8 | 14.6 ± 3.1 | 14.4 ± 2.1 | 1.14 | 3,39 | NS | 15 |
| Number of awakenings | 0.8 ± 0.3 | 0.7 ± 0.3 | 0.7 ± 0.2 | 1.3 ± 0.4 | 1.46 | 3,39 | NS | 15 |
| Sleep (h) | 7.2 ± 0.2 | 7.4 ± 0.2 | 7.5 ± 0.2 | 7.3 ± 0.3 | 0.36 | 3,39 | NS | 15 |
| Number of complaints | 1.9 ± 0.7 | 2.6 ± 1.0 | 1.5 ± 0.4 | 1.8 ± 0.6 | 0.60 | 3,39 | NS | 15 |
Abbreviations are as indicated in Table 1 Significant drug–placebo differences are indicated by.
P < 0.017;
P < 0.025. High scores on the subjective driving quality indicate better driving performance.
Subjective measures
On day 1 and day 8 sleep quality of the night before testing was comparable in all conditions.
Prior to the driving tests, subjects did not estimate their driving ability to be affected by any of the treatments on day 1 or day 8. At the end of the tests on day 1, however, subjects did notice that their driving performance had been impaired (F3,39 = 4.55; P = 0.008). In line with objective measures of driving performance, subjects rated their driving quality in the mequitazine and dexchlorpheniramine conditions as worse than after placebo (mean −12.8; CI −24.2, −2.7; F1,39 = 6.4; P < 0.025 and mean −16.4; CI −27.8, −6.3; F1,39 = 10.2; P < 0.017, respectively).
Discussion
Results of this study show that mequitazine 10 mg and dexchlorpheniramine Repetab 6 mg produced driving impairment on the first day of treatment. These results were expected and support our previous findings that single doses of these drugs can have impairing effects on actual driving [25]. The magnitude of the driving impairment found after single doses of mequitazine 10 mg and dexchlorpheniramine Repetab 6 mg is comparable to what has been found for second-generation antihistamines such as acrivastine 8 mg and mizolastine 10 and 20 mg [11, 12]. The present study also showed, however, that these effects disappear within 8 days of treatment.
Although the effects on driving found for mequitazine confirm our previous results, they are not supported by two earlier studies. Blamoutier (1978) and Hindmarch (1986) concluded that mequitazine 5 and 10 mg do not produce acute daytime drowsiness [31, 32]. However, these investigators used subjective measures and cognitive tests, so their results are in fact in line with those of our present and previous studies, showing no significant impairment of mequitazine on performance in psychomotor and cognitive tests or on subjective measures of sedation. This might indicate that these tests are not good predictors of driving ability [33, 34], or that the effects of these antihistamines were too subtle to be picked up by these tests.
Although dexchlorpheniramine in immediate release formulations (2 and 4 mg) has considerable sedative effects [35, 36], results from this study suggest that these effects can be reduced by a controlled release formulation. Similarly, it has been shown that controlled release formulations of triprolidine also reduce maximal levels of impairment [7, 14]. Therefore, it seems that controlled release formulations are a good way to diminish the sedative effects of antihistamines.
Cetirizine 10 mg had no effects on driving in the present study. Previous studies using the same driving test, however, demonstrated small but significant acute impairing effects after single doses of cetirizine 10 mg [26, 28]. The main difference between the latter studies and the present one is the time of testing with respect to drug administration. Ramaekers, Uiterwijk and O'Hanlon (1992) and Vermeeren, Ramaekers and O'Hanlon (2002) [26, 28] assessed the effects between 2 and 3 h after ingestion, whereas in the present study the effects of cetirizine on driving performance were tested between 1 and 2 h after ingestion (i.e. at Tmax). A previous study [15] using the same test also failed to find significant effects of cetirizine between 1 and 2 h after dosing. Together, these results suggest that there might be a delay between peak plasma concentrations and maximal effect on performance in the highway driving test. However, because of the impairment demonstrated in two previous driving studies and on psychomotor and subjective measures in other studies, it can not be concluded that cetirizine is always free of sedating effects [26–28, 37].
The clinical relevance of performance changes in the highway driving test has previously been determined by establishing the relationship between blood alcohol concentration (BAC) and SDLP [38]. Results showed that a BAC of 0.5 mg ml−1 is associated with an average increase in SDLP of 2.2 cm. Since a BAC of 0.5 mg/ml is associated with a significantly higher risk of traffic accidents [39], an SDLP increase of about 2.2 cm is considered clinically relevant. On the first day of treatment mequitazine and dexchlorpheniramine increased SDLP by 2.5 and 2.0 cm, respectively. In a previous study, however, mequitazine 10 mg caused an acute SDLP increase of 1.0 cm and dexchlorpheniramine an increase of 2.4 cm [25]. Although these results seem inconclusive on the degree of impairment caused by mequitazine and dexchlorpheniramine, they show that some individuals are prone to the sedating effects of these antihistamines and will show signs of impaired driving.
After 8 days of treatment, no treatment caused impairment in driving performance. The fact that the acute impairing effect of these antihistamines disappears after repeated doses has been found previously for other antihistamines and is probably due to tolerance [7, 10, 19]. Interestingly, a similar tolerance for the effects of H1-antagonism has been shown with tricyclic antidepressants, whose sedative effects are also mainly caused by H1-receptor blockade [33]. The mechanism whereby tolerance of the impairing effects of antihistamines develops, however, is still unclear.
As reported previously [28], subjects were not aware of the performance impairment caused by antihistamines. On the first day of mequitazine and dexchlorpheniramine treatment subjects were aware of their impaired driving only after the test was completed, but were unable to predict the impairment beforehand. In practice, this means that patients treated with antihistamines may be confident about their driving ability, whereas in fact they pose a considerable risk to traffic safety. Therefore, drug labels should warn explicitly of the impairing effects of sedating antihistamines and advise patients to be particularly careful at the beginning of treatment even if they feel capable of driving.
In conclusion, this study showed that mequitazine 10 mg and dexchlorpheniramine Repetab 6 mg can produce mild but clinically relevant effects on driving performance on the first day of treatment. After 8 days of treatment, the impairing effect of mequitazine and dexchlorpheniramine had disappeared.
Acknowledgments
This study was sponsored by Pierre Fabre Médicament, France. We thank Dr M. van Lansbergen, Dr I. van Maris and Dr N. Muntjewerff for their respective contributions to this study.
References
- 1.Simons FER, Simons KJ. The pharmacology and use of H1-receptor-antagonist drugs. N Engl J Med. 1994;330:1663–70. doi: 10.1056/NEJM199406093302307. [DOI] [PubMed] [Google Scholar]
- 2.Passalacqua G, Bousquet J, Bachert C, Church MK, Bindsley-Jensen C, Nagy L, Szemere P, Davies RJ, Durham SR, Horak F, Kontou-Fili K, Malling HJ, van Cauwenberge P, Canonica GW. The clinical safety of H1-receptor antagonists. An EAACI position paper. Allergy. 1996;51:666–75. doi: 10.1111/j.1398-9995.1996.tb02109.x. [DOI] [PubMed] [Google Scholar]
- 3.Burton WN, Conti DJ, Chen CY, Schultz AB, Edington DW. The impact of allergies and allergy treatment on worker productivity. J Occup Environ Med. 2001;43:64–71. doi: 10.1097/00043764-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Gilmore TM, Alexander BH, Mueller BA, Rivara FP. Occupational injuries and medication use. Am J Ind Med. 1996;30:234–9. doi: 10.1002/(SICI)1097-0274(199608)30:2<234::AID-AJIM16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Nolen TM. Sedative effects of antihistamines: safety, performance, learning, and quality of life. Clin Ther. 1997;19:39–55. doi: 10.1016/s0149-2918(97)80071-9. [DOI] [PubMed] [Google Scholar]
- 6.Hindmarch I, Shamsi Z. The effects of single and repeated administration of ebastine on cognition and psychomotor performance in comparison to triprolidine and placebo in healthy volunteers. Curr Med Res Opin. 2001;17:273–81. [PubMed] [Google Scholar]
- 7.O'Hanlon JF, Ramaekers JG. Antihistamine effects on actual driving performance in a standard test: a summary of Dutch experience, 1989–94. Allergy. 1995;50:234–42. doi: 10.1111/j.1398-9995.1995.tb01140.x. [DOI] [PubMed] [Google Scholar]
- 8.Moskowitz H, Burns MB. Effects of terfenadine, diphenhydramine, and placebo on skills performance. Cutis. 1988;42:14–8. [PubMed] [Google Scholar]
- 9.Hindmarch I, Shamsi Z. Antihistamines: models to assess sedative properties, assessment of sedation, safety and other side-effects. Clin Exp Allergy. 1999;29:133–42. doi: 10.1046/j.1365-2222.1999.0290s3133.x. [DOI] [PubMed] [Google Scholar]
- 10.Kay GG, Berman B, Mockoviak SH, Morris CE, Reeves D, Starbuck V, Sukenik E, Harris AG. Initial and steady-state effects of diphenhydramine and loratadine on sedation, cognition, mood, and psychomotor performance. Arch Intern Med. 1997;157:2350–6. [PubMed] [Google Scholar]
- 11.Vuurman EF, Uiterwijk MM, Rosenzweig P, O'Hanlon JF. Effects of mizolastine and clemastine on actual driving and psychomotor performance in healthy volunteers. Eur J Clin Pharmacol. 1994;47:253–9. doi: 10.1007/BF02570505. [DOI] [PubMed] [Google Scholar]
- 12.Ramaekers JG, O'Hanlon JF. Acrivastine, terfenadine and diphenhydramine effects on driving performance as a function of dose and time after dosing. Eur J Clin Pharmacol. 1994;47:261–6. doi: 10.1007/BF02570506. [DOI] [PubMed] [Google Scholar]
- 13.Riedel WJ, Ramaekers G, Uiterwijk MM, O'Hanlon JF. Higher Doses of Terfenadine and Loratadine: Acute and Subchronic Effects on Psychomotor and Actual Driving Performance. Maastricht: Institute for Drugs, Safety and Behavior, University of Limburg; 1990. [Google Scholar]
- 14.Brookhuis KA, De Vries G, De Waard D. Acute and subchronic effects of the H1-histamine receptor antagonist ebastine in 10, 20 and 30 mg dose, and triprolidine 10 mg on car driving performance. Br J Clin Pharmacol. 1993;36:67–70. doi: 10.1111/j.1365-2125.1993.tb05894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkerts ER, Van Willigenburg AP, Van Laar MW, Maes RA. Does cetirizine belong to the new generation of antihistamines? An investigation into its acute and subchronic effects on highway driving, psychometric test performance and daytime sleepiness. Human Psychopharmacol Clin Exp. 1992;7:227–38. [Google Scholar]
- 16.Robbe HWJ, O'Hanlon JF. Effects of Acrivastine, Acrivastine plus Pseudoephedrine and Triprolidine on Highway Driving. Maastricht: Institute for Drugs, Safety and Behavior University of Limburg; 1990. [Google Scholar]
- 17.Verster JC, de Weert AM, Bijtjes SI, Aarab M, van Oosterwijck AW, Eijken EJ, Verbaten MN, Volkerts ER. Driving ability after acute and sub-chronic administration of levocetirizine and diphenhydramine: a randomized, double-blind, placebo-controlled trial. Psychopharmacology. 2003;169:84–90. doi: 10.1007/s00213-003-1462-6. [DOI] [PubMed] [Google Scholar]
- 18.Vermeeren A, O'Hanlon JF. Fexofenadine's effects, alone and with alcohol, on actual driving and psychomotor performance. J Allergy Clin Immunol. 1998;101:306–11. doi: 10.1016/S0091-6749(98)70240-4. [DOI] [PubMed] [Google Scholar]
- 19.Richardson GS, Roehrs TA, Rosenthal L, Koshorek G, Roth T. Tolerance to daytime sedative effects of H1 antihistamines. J Clin Psychopharmacol. 2002;22:511–5. doi: 10.1097/00004714-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Seppala T, Nuotto E, Korttila K. Single and repeated dose comparison of three antihistamines and phenylpropanolamine: psychomotor performance and subjective appraisals of sleep. Br J Clin Pharmacol. 1981;12:179–88. doi: 10.1111/j.1365-2125.1981.tb01198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verster JC, Volkerts ER, van Oosterwijck AW, Aarab M, Bijtjes SI, De Weert AM, Eijken EJ, Verbaten MN. Acute and subchronic effects of levocetirizine and diphenhydramine on memory functioning, psychomotor performance, and mood. J Allergy Clin Immunol. 2003;111:623–7. doi: 10.1067/mai.2003.63. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson AN, Stone BM. The H1-antagonist mequitazine: studies on performance and visual function. Eur J Clin Pharmacol. 1983;25:563–6. doi: 10.1007/BF00542129. [DOI] [PubMed] [Google Scholar]
- 23.Muler H, Blum F. Essai comparé de deux antihistaminiques: Mequitazine et Dexchlorpheniramine. Ann Otolaryngol Chir Cervicofac. 1978;95:433–9. [PubMed] [Google Scholar]
- 24.Caille EJ. Etude comparative en double aveugle de la méquitazine, de la dexchlorphéniramine et d'un placebo sur la vigilance. Gazette Médicale France. 1979;86:3737–44. [Google Scholar]
- 25.Theunissen EL, Vermeeren A, van Oers ACM, van Maris I, Ramaekers JG. A dose ranging study of the effects of mequitazine on actual driving, memory and psychomotor performance as compared to dexchlorpheniramine, cetirizine and placebo. Clin Exp Allergy. 2004;34:250–8. doi: 10.1111/j.1365-2222.2004.01874.x. [DOI] [PubMed] [Google Scholar]
- 26.Ramaekers JG, Uiterwijk MM, O'Hanlon JF. Effects of loratadine and cetirizine on actual driving and psychometric test performance, and EEG during driving. Eur J Clin Pharmacol. 1992;42:363–9. doi: 10.1007/BF00280119. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson AN, Turner C. Central effects of the H1-antihistamine, cetirizine. Aviat Space Environ Med. 1998;69:166–71. [PubMed] [Google Scholar]
- 28.Vermeeren A, Ramaekers JG, O'Hanlon JF. Effects of emedastine and cetirizine, alone and with alcohol, on actual driving of males and females. J Psychopharmacol. 2002;16:57–64. doi: 10.1177/026988110201600104. [DOI] [PubMed] [Google Scholar]
- 29.Vuurman EFPM, Rikken GH, Muntjewerff ND, de Halleux F, Ramaekers JG. Effects of desloratadine, diphenhydramine, and placebo on driving performance and psychomotor performance measurements. Eur J Clin Pharmacol. 2004;60:307–13. doi: 10.1007/s00228-004-0757-9. [DOI] [PubMed] [Google Scholar]
- 30.Mulder-Hajonides van der Meulen WREH. Proceedings of International European Sleep Congress. Amsterdam: Elsevier; 1981. Measurments of subjective sleep quality. [Google Scholar]
- 31.Hindmarch I, Easton JC. A placebo-controlled assessment of mequitazine and astemizole in tests of psychomotor ability. Int J Clin Pharmacol Res. 1986;6:457–64. [PubMed] [Google Scholar]
- 32.Blamoutier J. Comparative trial of two antihistamines, mequitazine and brompheniramine. Curr Med Res Opin. 1978;5:366–70. doi: 10.1185/03007997809111899. [DOI] [PubMed] [Google Scholar]
- 33.Ramaekers JG. Antidepressants and driver impairment: empirical evidence from a standard on-the-road test. J Clin Psychiatry. 2003;64:20–9. [PubMed] [Google Scholar]
- 34.Verster JC, Volkerts ER, Verbaten MN. The predictive value of driving-related laboratory tests and subjective assessments to on-the-road driving ability. J Psychopharmacol. 2002;3:A62. [Google Scholar]
- 35.Kamei H, Noda Y, Ishikawa K, Senzaki K, Muraoka I, Hasegawa Y, Hindmarch I, Nabeshima T. Comparative study of acute effects of single doses of fexofenadine, olopatadine, d-chlorpheniramine and placebo on psychomotor function in healthy volunteers. Human Psychopharmacol. 2003;18:611–8. doi: 10.1002/hup.538. [DOI] [PubMed] [Google Scholar]
- 36.Okamura N, Yanai K, Higuchi M, Sakai J, Iwata R, Ido T, Sasaki H, Watanabe T, Itoh M. Functional neuroimaging of cognition impaired by a classical antihistamine, d-chlorpheniramine. Br J Clin Pharmacol. 2000;129:115–23. doi: 10.1038/sj.bjp.0702994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tashiro M, Sakurada Y, Iwabuchi K, Mochizuki H, Kato M, Aoki M, Funaki Y, Itoh M, Iwata R, Wong DF, Yanai K. Central effects of fexofenadine and cetirizine: measurement of psychomotor performance, subjective sleepiness, and brain histamine H1-receptor occupancy using 11C-doxepin positron emission tomography. J Clin Pharmacol. 2004;44:890–900. doi: 10.1177/0091270004267590. [DOI] [PubMed] [Google Scholar]
- 38.Louwerens JW, Gloerich ABM, de Vries G, Brookhuis KA, O'Hanlon JF. The relationship between drivers' blood alcohol concentration (BAC) and actual driving performance during high speed travel. In: Noordzij PC, Roszbach R, editors. International Congress on Alcohol, Drugs and Traffic Safety, T86. Amsterdam: Exerpta Medica; 1987. pp. 183–6. [Google Scholar]
- 39.Borkenstein RF, Crowther RF, Shumate RP, Ziel WB, Zylman R. The role of the drinking driver in traffic accidents. Blutalkohol. 1974;11:1–131. [Google Scholar]