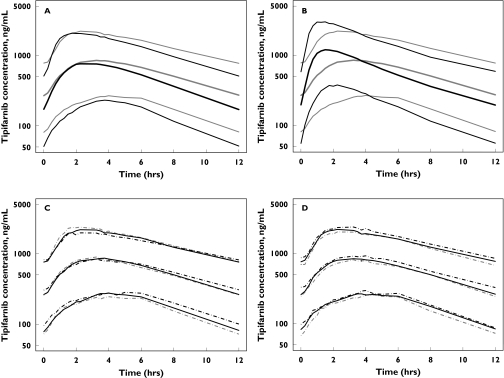
Figure 5.
Simulated steady-state plasma concentration vs. time profiles of tipifarnib 600 mg twice daily in healthy ( ) and cancer (
) and cancer ( ) subjects receiving a solid formulation (A), in cancer subjects receiving solid (
) subjects receiving a solid formulation (A), in cancer subjects receiving solid ( ) and liquid formulations (
) and liquid formulations ( ) (B), and the effect of body weight <60 kg (
) (B), and the effect of body weight <60 kg ( ), 60–80 kg (
), 60–80 kg ( ), >80 kg (
), >80 kg ( ) (C) and bilirubin <7.5 mM (
) (C) and bilirubin <7.5 mM ( ), 7.5–15 mM (
), 7.5–15 mM ( ), >15 mM (
), >15 mM ( ) concentrations (D) in cancer subjects receiving a solid formulation. Lines represent 10 (lower), 50 (middle) and 90 (upper) quantiles of simulated plasma concentration
) concentrations (D) in cancer subjects receiving a solid formulation. Lines represent 10 (lower), 50 (middle) and 90 (upper) quantiles of simulated plasma concentration