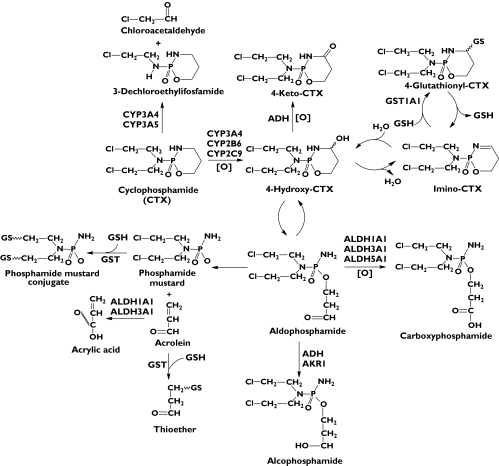
Figure 1.
The metabolic pathways of cyclophosphamide (CTX) in humans. As a prodrug, CTX is activated by hepatic CYP2B6, CYP3A4 and CYP2C9 to form 4-OH-CTX. 4-OH-CPA is in equilibrium with its tautomer aldophosphamide which can decompose spontaneously by β-elimination to result in cytotoxic phosphoramide mustard and the toxic by-product acrolein. Acrolein is detoxified by glutathione S-transferases (GSTs). Alternatively, 4-OH-CTX is detoxified by aldehyde dehydrogenases (ALDH1A, ALDH3A1 and ALDH5A) to generate nontoxic carboxyphosphamide. 4-OH-CTX is also oxidized by alcohol dehydrogenase (ADH) to form nontoxic 4-keto-CTX. Furthermore, 4-OH-CTX undergoes reversible dehydration to result in immino-CTX that is further conjugated with intracellular GSH, giving rise to 4-glutathionyl-CTX, a substrate for multidrug resistance associated protein 2. Moreover, CTX can be converted to 3-dechloroethylifosfamide to a minor extent by CYP3A-catalysed side chain N-dechloroethylation with the production of the toxic by-product chloroacetaldehyde