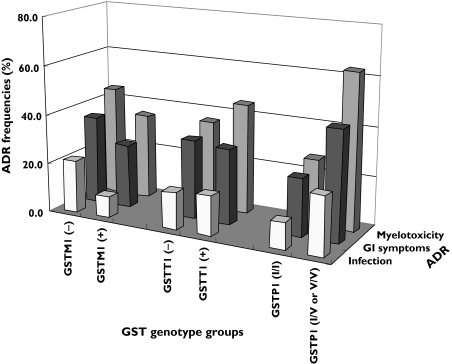
Figure 2.
The incidence of short-term (2-week) side-effects, including myelotoxicity, gastrointestinal (GI) symptoms, and infection with different GSTM1, GSTT1 and GSTP1 genotypes in SLE patients receiving pulsed high-dose (1.0 g) cyclophosphamide (CTX) therapy. The incidence of myelotoxicity and GI symptoms was significantly higher in patients with the GSTP1-*105I/V or GSTP1-105 V/V genotype than in patients with the GSTP1 wildtype genotype (P < 0.001 and P < 0.05, respectively)