Figure 9.
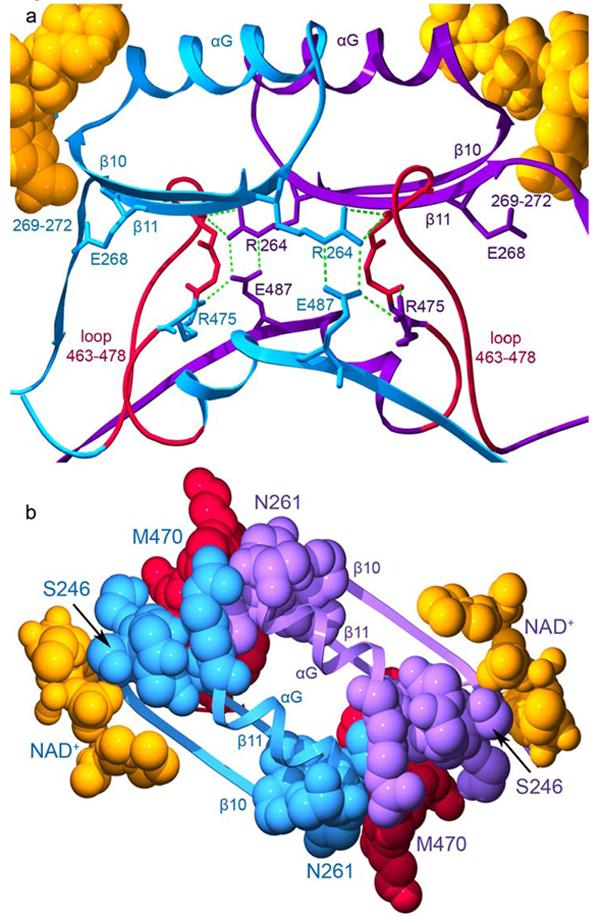
The interactions across the dimer interface contributed by residues 463-478. The structure of the wild-type ALDH2 enzyme with coenzyme-bound (PDB ID 1O02) is used for this representation. Subunits A (blue) and B (violet) are shown. a) The loop comprised of residues 463-478 is shown in red for both subunits with the side chain for residue 475 colored according to its subunit. Hydrogen bonds are represented by green dashed lines. b) Contacts among residues 463-478, the αG helices, and β-strands at the interface. The view in this panel is rotated 90° about a horizontal axis with respect to panel a. The elements of secondary structure are labeled. Residues 246 and 261, which mark the beginning and end of αG are labeled as is residue 470 within the loop which contacts these regions. The bound coenzyme molecules are shown using space-filling atoms and are colored gold.
