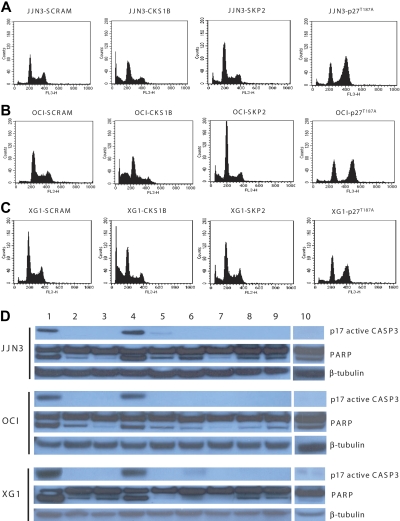
Figure 3.
Silencing of CKS1B induces apoptosis by activating caspase-3 and PARP. (A) Cell cycle distribution and apoptosis were evaluated by flow cytometry analysis performed 5 days after lentiviral infection in JJN3 cells expressing a scrambled sequence (SCRAM), CKS1B shRNA, SKP2 shRNA, or p27T187A cDNA. Note that silencing of CKS1B induced cell cycle arrest in G0-G1 phase and dramatic increase in the percentage of cells with sub-G0 DNA content (indicative of apoptosis). SKP2 shRNA deregulated the G1-S transition more strongly than CKS1B shRNA, but caused a modest increase of apoptotic cells; the overexpression of p27T187A had no affect on apoptosis, but resulted in a significantly higher percentage of cells in G2-M phase. The same analysis shown in panel A was performed in (B) OCI-MY5 and (C) XG-1. (D) Caspase-3 (active form, p17) and PARP activation was evaluated by Western blot analysis performed in the same aliquots of cells used in panels A-C. The following conditions were analyzed: (1) positive control (myeloma cells treated with bortezomib at 10 nM for 24 hours); (2) positive control + Z-VAD-fmk; (3) negative control (myeloma cells without any treatment); (4) CKS1B shRNA; (5) CKS1B shRNA + Z-VAD-fmk; (6) SCRAM; (7) SCRAM + Z-VAD-fmk; (8) empty vector; (9) p27T187A cDNA; (10) SKP2 shRNA. Note that the activation of caspase-3 and PARP in cells expressing CKS1B shRNA could be abrogated by pretreatment with the pan-caspase inhibitor Z-VAD-fmk, indicating a caspase-dependent mechanism of apoptosis.