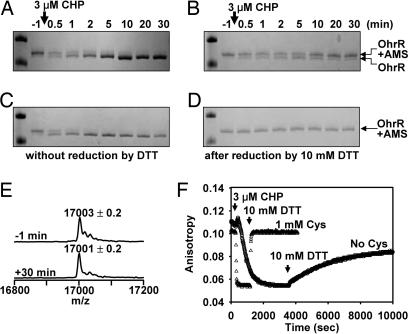
Fig. 3.
Kinetics of OhrR oxidation and re-reduction in the absence of Cys. (A–D) AMS reactivity of OhrR after CHP treatment was monitored as a function of time. (A) OhrR oxidized in the absence of Cys. The accumulation of the faster migrating band as a function of time indicates a loss of AMS reactivity. (B) Samples shown in A were treated with DTT for 60 min before AMS alkylation. Oxidized OhrR was largely restored to an AMS-reactive form, consistent with the OhrR sulfenamide as the major product. (C and D) Shown are the parallel reactions in the presence of 1 mM Cys. Only the region of the gel corresponding to the OhrR-AMS and unalkylated OhrR bands is shown (see SI Fig. 9 for an image of the entire gel). (E) The molecular mass of OhrR was monitored by ESI-MS before and 30 min after CHP (3 μM) treatment (see SI Fig. 10 for the time series data). (F) Comparison of OhrR inactivation (by CHP) and reactivation (by DTT) in reactions with or without Cys.