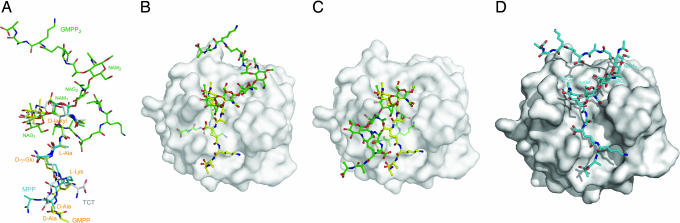
Fig. 3.
Structural comparison between PGRP-bound PGN analogs in crystal structures and unbound GMPP2 in solution. (A) Conformational comparison of GMPP, MPP, TCT, and GMPP2. GMPP, MPP, and TCT are from crystal structures of complexes with human PGRP-IβC, human PGRP-IαC (16), and Drosophila PGRP-LE (27), respectively; GMPP2 is from the unliganded NMR structure (17). The structures are superposed through the pyranose ring of NAM (for MPP, GMPP, and GMPP2) or NAM(1,6-anhydro) (for TCT). (B) Superposition of unbound GMPP2 onto GMPP in the PGRP-IβC–GMPP complex. GMPP and GMPP2 are shown in ball-and-stick representations, with carbon atoms in yellow and green, respectively, nitrogen atoms in blue, and oxygen atoms in red. Of the two GMPP units in GMPP2, the first unit, comprising the NAG1-NAM1 disaccharide, is superposed onto GMPP in the complex. The peptide stem of GMPP2 attached to NAM1 is buried within PGRP-IβC and is shown in pale green. (C) Alternative superposition of unliganded GMPP2 onto GMPP bound to PGRP-IβC. In this case, the second GMPP unit of GMPP2, containing NAG2-NAM2, is superposed onto GMPP in the PGRP-IβC–GMPP structure. The peptide stem of GMPP2 attached to NAM2, shown in pale green, is buried inside PGRP-IβC. (D) Modeled PGRP-IβC–GMPP2 structure.