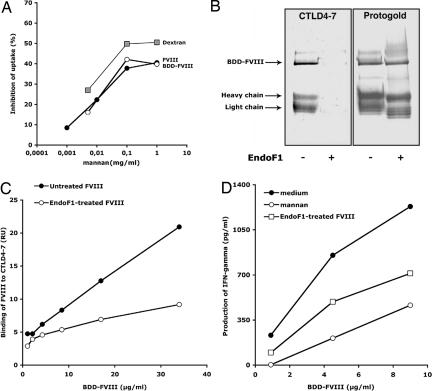
Fig. 4.
Exposed mannose residues located outside the B domain play a significant role in FVIII endocytosis by DC leading to T cell activation. (A) DC were preincubated with mannan before the addition of FVIII (40 μg/ml,143 nM, filled circles) and BDD-FVIII (24.31 μg/ml,143 nM, open circles), or dextran-FITC (50 μg/ml). Antigen uptake was analyzed by flow cytometry. Percentage inhibition was calculated for each condition with respect to the condition without mannan. Representative of two individual experiments. (B) Western blot analysis of native or EndoF1-treated BDD-FVIII (3.7 μg) revealed by using Protogold or CTLD4–7-Fc using an alkaline phosphatase-conjugated anti-human IgG. The light chain (LC) and heavy chain (HC) were identified upon blotting with LC- and HC-specific monoclonal anti-FVIII IgGs (data not shown). (C) Binding of native or EndoF1-treated BDD-FVIII to CTLD4–7-Fc was assessed by surface plasmon resonance. Depicted values represent the resonance units (RU) measured 250 s after the end of the injection on a CTLD4–7-Fc-coated channel, from which the background binding to an uncoated channel has been subtracted. (D) Reduced activation of T cells upon EndoF1 treatment of BDD-FVIII. Results depict one representative of three independent experiments. Yields of IFN-γ varied with different batches of D9E9 and with the different sources of human DC used in the different experiments. To statistically compare the three sets of experiments, the production of IFN-γ was normalized with respect to the maximum value obtained in each individual experiment. Differences in normalized levels of T cell activation were statistically significant between “medium” and “EndoF1-treated” (P < 0.0001, ANOVA and Fisher's protected least-significant difference, data not shown).