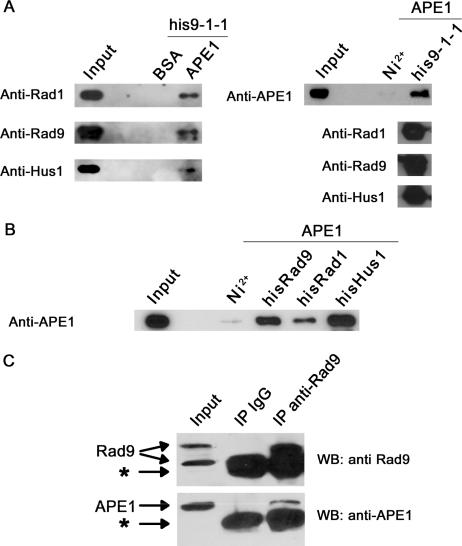
Figure 5.
The 9-1-1 complex physically interacts with APE 1 in vitro and in vivo. (A) Direct interaction of the 9-1-1 complex with APE 1 in vitro. APE 1-sepharose pulldowns (right panel) were performed by incubating APE 1-sepharose beads, or BSA-sepharose as a negative control, together with purified his9-1-1 complex as described in ‘Materials and Methods’. His9-1-1 pulldowns (left panel) were performed by incubating purified his9-1-1 complex with purified APE 1 and subsequent binding to Ni2+ beads. Five percent of the pulldown were used to check the presence of all the subunits of the 9-1-1 complex (his-Rad9, Rad1 and Hus1), and the remaining sample was used to detect co-precipitated APE 1 by SDS-PAGE followed by western blot analysis. Input represents 2% of the total amount of interacting protein used in the pulldown experiments. (B) Physical interaction of APE 1 with the 9-1-1 complex subunits. His-pulldowns were performed by using the individual subunits his-Rad1, his-Rad9, and his-Hus1 as described in A. (C) The 9-1-1 complex interacts with APE1 in vivo. Immunoprecipitation experiments were performed as described in ‘Material and Methods’ by incubating 293T total cell extracts with anti-human Rad9 antibody. Presence of immuno-precipitated Rad9 and co-precipitated APE1 were analyzed by SDS-PAGE followed by western blot analysis. The lane IP IgG contains the control immunoprecipitation performed in the presence of un-immunized rabbit IgG. Input represents 5% of the amount of extract used for immunoprecipitation. Arrows indicate the positions of endogenous APE1 and Rad9. The bands indicated by asterisks correspond to the antibody heavy and light chain respectively.