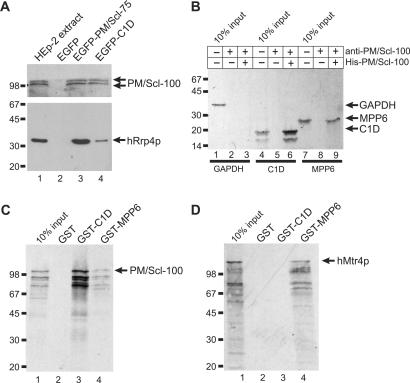
Figure 2.
Association of C1D, MPP6 and hMtr4p with components of the exosome. (A) Co-immunoprecipitations were performed using anti-EGFP antibodies and extracts of HEp-2 cells transiently transfected with expression constructs for either EGFP alone (lane 2), EGFP-PM/Scl-75 (lane 3) or EGFP-C1D (lane 4). In the first lane, total extract from non-transfected HEp-2 cells was separated. The precipitated proteins were analyzed by western blotting, using anti-PM/Scl-positive patient serum R212 (upper part of the blot) or a monoclonal antibody to hRrp4p (lower part of the blot). Arrows indicate the positions of PM/Scl-100 and hRrp4p. The positions of molecular weight markers are indicated on the left. (B) Bacterially expressed, recombinant His-tagged PM/Scl-100 was immobilized using anti-PM/Scl-100 antibodies and incubated with either 35S-labeled, in vitro translated GAPDH (lane 1), C1D (lane 4) or MPP6 (lane 7). Co-precipitated proteins were analyzed by SDS-PAGE and autoradiography. The positions of these proteins are indicated with arrows, and the positions of molecular weight markers are indicated on the left. Lanes 3, 6 and 9 show proteins co-precipitated with His-PM/Scl-100. In lanes 2, 5 and 8, material from control incubations, in which no recombinant His-PM/Scl-100 was added, was analyzed. (C) Glutathione-Sepharose beads were used to precipitate GST (lane 2), GST-C1D (lane 3) or GST-MPP6 (lane 4), which were incubated with 35S-labeled, in vitro translated PM/Scl-100. After precipitation, bound PM/Scl-100 was analyzed by SDS-PAGE and autoradiography. In lane 1, 10% of the amount of labeled PM/Scl-100 used per incubation was loaded. On the left, the positions of molecular weight markers are indicated. (D) Similar experiments as described in (C), but now with 35S-labeled, in vitro translated hMtr4p instead of PM/Scl-100.