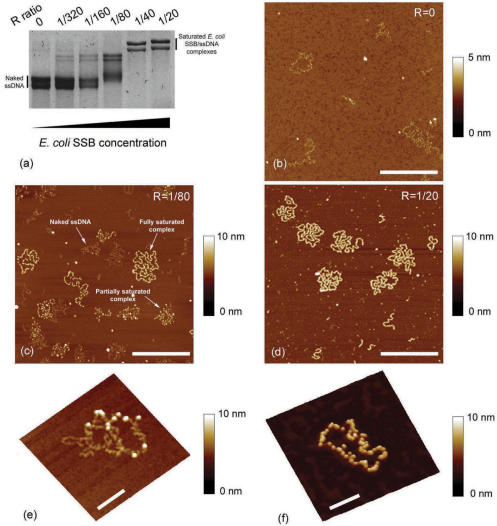
Figure 2.
(a) Agarose gel electrophoresis of M13 ssDNA–E. coli SSB complexes formed in spermidine buffer Tris 20 mM pH 7.5, NaCl 20 mM, SpdCl3 50 µM with increasing E. coli SSB protein concentration: free ssDNA (lane 1), R = 1/320 (lane 2), R = 1/160 (lane 3), R = 1/80 (lane 4), R = 1/40 (lane 5) and R = 1/20 (lane 6). (b) AFM image of free M13ssDNA. (c) AFM image of M13 ssDNA–E. coli SSB complexes associated to lane 4. Free ssDNA, partly formed complex and nearly fully saturated complex coexist and are a typical feature of cooperative binding of E. coli SSB to ssDNA. (d) AFM image of fully saturated M13 ssDNA–E. coli SSB complexes associated to lane 6 (scale bars 500 nm). (e) Zoom on M13 ssDNA–E. coli SSB complexes (R = 1/320 and scale bar 100 nm) at which few SSB proteins can be distinguished. (f) Zoom on a nearly fully saturated M13 ssDNA–E. coli SSB complex (R = 1/40 and scale bar 100 nm). Even at high R value, single SSB protein can be resolved on the complex thread.