Figure 2.
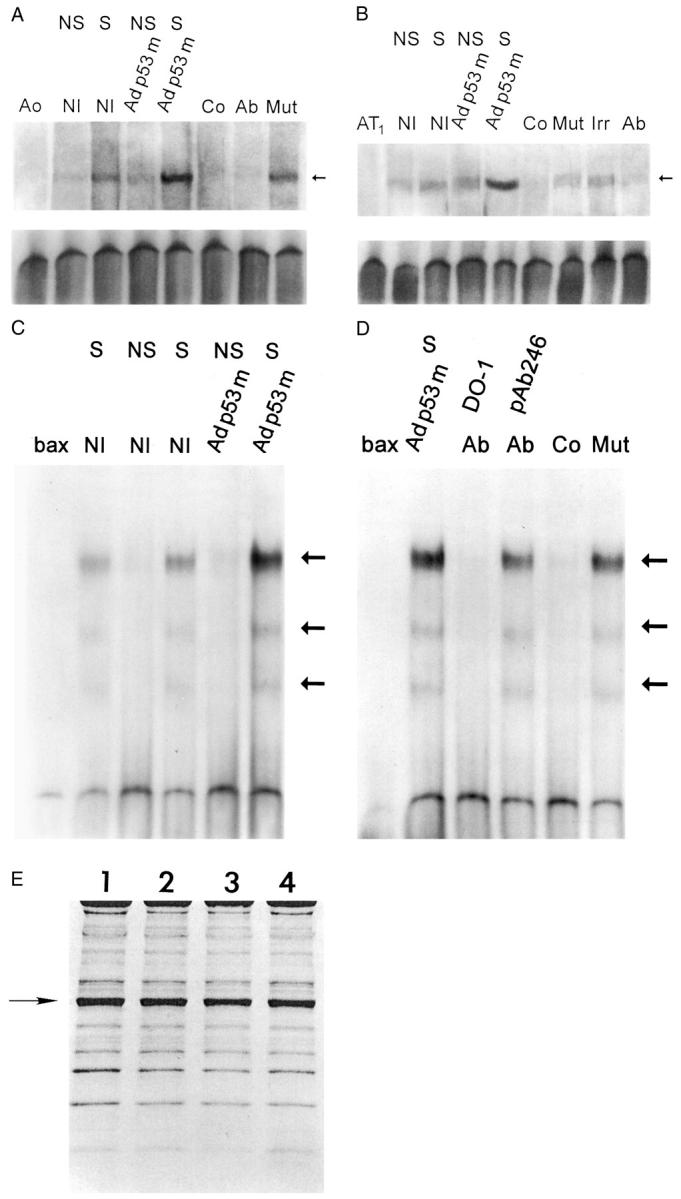
p53 binding to the promoter of Aogen (A), AT1 receptor (B), and bax (C and D). Nuclear extracts were obtained from NS and S noninfected (NI), and Adp53m-infected myocytes. Arrows in A, B, C, and D indicate the position of the p53-shifted bands. p53 bands were subjected to competition with excess of unlabeled self-oligonucleotides (Co) and with p53 antibodies (Ab); pAb 240 was used for Aogen and AT1 assays whereas DO-1 and pAb 246 were used for bax assay. Addition of an irrelevant antibody (Irr) or mutated form of Aogen, AT1, or bax (Mut) did not interfere with p53 binding. Ao, Aogen probe; AT1, AT1 probe; bax, bax probe (n = 3 in all cases). OD (A) in noninfected myocytes: NS = 1.50 ± 0.27; S = 3.76 ± 0.48; Adp53m-infected myocytes: NS = 2.02 ± 0.36; S = 10.3 ± 1.5. OD (B) in noninfected myocytes: NS = 2.15 ± 0.56; S = 4.00 ± 0.82; Adp53m-infected myocytes: NS = 4.23 ± 0.91; S = 19.9 ± 1.7. OD (C and D) in noninfected myocytes: NS = 0.28 ± 0.15; S = 3.3 ± 0.61; Adp53m-infected myocytes: NS = 0.32 ± 0.14; S = 11.59 ± 1.3. E: Pattern of proteins corresponding to nuclear preparations for mobility shift assay is shown by Coomassie blue. OD of the actin band (arrow) was significantly reduced and was consistent throughout, confirming that the level of cytoplasmic contamination was similar in the nuclear preparations: 1 = noninfected NS myocytes; 2 = noninfected S myocytes; 3 = Adp53m-infected NS myocytes; 4 = Adp53m-infected S myocytes.
