Abstract
Microcystic adenoma and cysts of the pancreas occur sporadically or as a part of von Hippel-Lindau (VHL) disease. The pathology of pancreatic cystic disease in VHL patients has not been well characterized. Furthermore, it is presently unknown whether the alteration of the VHL gene is responsible for the development of the entire spectrum of pancreatic serous cystic lesions. We performed a histopathological analysis of 21 cysts and 98 microcystic adenomas in nine VHL patients with a known germline mutation. In addition, PCR-amplified DNA from 27 pancreatic cystic lesions in three informative patients was studied for allelic deletions with polymorphic markers spanning the VHL gene locus. In all patients, pancreatic lesions were multiple: 21 benign serous cysts, 63 microscopic microcystic adenomas (size <0.4 cm), and 35 macroscopic microcystic adenomas (size >0.5 cm). The average number of lesions per patient was 2.1 benign cysts (range, 0–8), 7.7 (1–37) microscopic microcystic adenomas, and 3 (0–21) macroscopic microcystic adenomas. All lesions showed similar histology and contained prominent fibrous stroma, clear and/or amphophilic, glycogen-rich epithelial cells, endothelial and smooth muscle cells. VHL deletions were detected in all types of pancreatic cystic lesions. The presence of VHL gene allelic deletions in the spectrum of multifocal pancreatic cystic lesions provides direct molecular evidence of their neoplastic nature and integral association with VHL disease. The histopathological and molecular data establish a serous cyst-microcystic adenoma continuum in the development of pancreatic cystic neoplasia in VHL disease.
von Hippel-Lindau (VHL) disease is an autosomal dominant disorder in that affected individuals are predisposed to develop a variety of neoplasms in multiple target organs. The lesions are frequently multiple in a given organ and include hemangioblastoma of the central nervous system, retinal angioma, endolymphatic sac tumor, renal cell carcinoma (RCC), pheochromocytoma, and cysts of the kidneys and epididymis. 1 Pancreatic neuroendocrine (islet cell) tumors are reported to occur with an incidence of 12 to 17% in VHL patients. 2-4 However, the most common manifestations of pancreatic disease are benign serous cysts (BC) and a microcystic (serous) adenoma (MCA), which occur in 35 to 75% of VHL patients. 5,6 MCA may also occur sporadically and is a benign tumor with cystic or solid architecture composed of cysts of various sizes lined by flattened or cuboidal glycogen-rich cells. 7-9 The origin of this cell remains unclear, although previous histological and ultrastructural studies suggested derivation from a centroacinar cell 7-9 or ductal cell. 10,11 Although VHL-associated cystadenoma has been reported in medical and radiology literature, the histopathology of pancreatic cystic disease in VHL patients has not been well characterized. 5-8,12-14
The VHL tumor suppressor gene has been localized to the chromosome 3p25.5 and identified in 1993. 15 The two-hit theory of Knudson 16 predicts that in a familial cancer syndrome such as VHL disease, the genotype of each neoplasm is determined by the presence of the inherited allele with a germline mutation and by the wild-type allele loss through allelic deletion. Molecular genetic studies of VHL disease-associated neoplasms such as central nervous system hemangioblastoma, 17,18 retinal angioma, 19 renal cysts and RCC, 20,21 pheochromocytoma, 22,23 pancreatic neuroendocrine tumor, 3 and endolymphatic sac tumor 24 have demonstrated loss of heterozygosity (LOH) at the VHL gene region. Although VHL allelic deletions have been reported in VHL-related MCA, 25 it is presently unknown whether the alteration of the VHL gene is responsible for development of the entire spectrum of pancreatic serous cystic lesions in VHL patients.
Materials and Methods
Patients
Nine VHL patients (3 female, 6 male; mean age, 42 years; range, 29–71 years) with pancreatic cystic lesions were selected from the group of familial VHL patients followed on the Institutional Review Board-approved protocol at the National Cancer Institute of the National Institutes of Health 4 (Table 1) ▶ . Patients had a documented germline mutation in the VHL gene, and in two VHL patients (nos. 6 and 9), theVHL germline mutation was detected in a close relative. Most patients were clinically asymptomatic, and their pancreatic cystic lesions were discovered incidentally during VHL screening by computed tomography or magnetic resonance imaging (CT/MRI), 26 histopathological evaluation of the pancreas adjacent to the surgically removed neuroendocrine (islet cell) tumor, 3 or autopsy.
Table 1.
Clinical, Histopathological and Genetic Data in Nine VHL Patients with Cystic Disease of the Pancreas
| Patient no. | Age/sex | Procedure | BC no. | MMCA no. | mMCA no. | VHL germline mutation | LOH at 3p25.5 | Other VHL tumors |
|---|---|---|---|---|---|---|---|---|
| 1* | 42 /M | Distal pancr | 3 | 6 | 0 | nt 703, C to T, Gln to stop, exon 3 | 3 BC and 6 mMCA | h |
| 2* | 44 /F | Distal pancr | 8 | 21 | 37 | nt 430, C to T, Pro to Ser, exon 1 | 1 BC, 2 mMCA and 4 MMCA | h, ra |
| 3 | 31 /M | Enucleation | 0 | 1 | 1 | Partial deletion | ND | h, ph, ra, rcc, rc, net |
| 4* | 34 /M | Panc/duod | 2 | 1 | 5 | Partial deletion | 2 BC, 5 mMCA and 1 MMCA | h, ph, ra, rc, net |
| 5 | 47 /F | Total pancr | 0 | 1 | 1 | nt 712, C to T, Arg to Trp, exon 3 | ND | h, ra, ph, rc |
| 6 | 42 /F | Total pancr | 2 | 1 | 6 | nt 393 del G, codon 137, exon 1, stop | ND | h, net |
| 7 | 29 /M | Partial pancr | 6 | 0 | 9 | nt 699 C to G, Cys to Trp, exon 3 | ND | h, ra, ph, rcc, net |
| 8 | 40 /M | Autopsy | 0 | 2 | 2 | NA | ND | h, ph, rcc |
| 9 | 71 /M | Autopsy | 0 | 2 | 2 | nt 430, C to T, Pro to Ser, exon 1 | ND | h, ra, ph, rcc, rc |
F, female; M, male; pancr, pancreatectomy; panc/duod, pancreaticoduodenectomy (Whipple’s procedure); BC, single benign cyst; mMCA, microscopic microcystic adenoma; MMCA, macroscopic microcystic adenoma; h, hemangioblastoma; ra, retinal angioma; ph, pheochromocytoma; rcc, renal cell carcinoma; rc, renal cysts; net, pancreatic neuroendocrine tumor; NA, not available; ND, not done.
*DNA from patients used for LOH study.
Tumors
Formalin-fixed, paraffin-embedded pancreatic tissue and 119 pancreatic cystic lesions were obtained from the files of the Laboratory of Pathology, National Cancer Institute. All lesions were examined grossly and microscopically. Lesions were evaluated on hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), PAS-diastase (PAS-D), and Masson’s trichrome stains. Immunohistochemistry stains for cytokeratins MAK 6 (1:2; Zymed, San Francisco, CA) and AE1/AE3 (1:300/1:100; Boehringer Mannheim, Indianapolis, IN), chromogranin A (1:1600; Boehringer Mannheim, Indianapolis, IN), CD31 (1:20), vimentin (1:40), and smooth muscle actin (SMA; 1:160; DAKO, Carpenteria, CA) were performed. Pancreatic lesions were arbitrarily classified based on architecture and size as i) single benign serous cyst (BC), ii) microscopic MCA (mMCA; <0.4 cm in size), or iii) macroscopic MCA (MMCA; >0.5 cm in size) (Table 1 ▶ , Figure 1 ▶ ).
Figure 1.
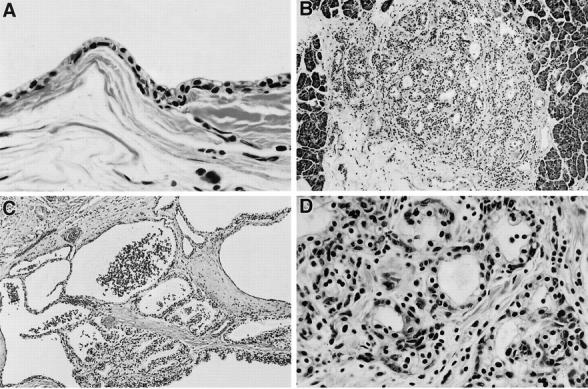
Histology of multiple pancreatic cystic lesions in VHL patients (H&E). A: Benign serous cyst (×400). B: Microscopic MCA (×200). C: Macroscopic MCA (×200). D: High power view of microscopic MCA (×400); epithelial cells intermixed with numerous endothelial cells that form small vessels, and stromal fibrosis.
Microdissection
Formalin-fixed, paraffin-embedded 5-μm tissue sections on glass slides from 27 pancreatic cystic lesions in three patients were used for LOH analysis. A modified microdissection procedure was performed under direct light microscopic visualization using a 30 gauge needle as previously described. 25,27 Cyst-lining epithelial cells were selectively procured for analysis. Because the endothelial cells were closely intermixed with the epithelial cells in pancreatic lesions under study (Figures 1 and 3) ▶ ▶ , we were unable to completely exclude endothelial cells during microdissection and LOH analysis. Stromal and smooth muscle cells were not used for analysis. Control samples were obtained from the matched normal pancreatic exocrine and endocrine tissue on the same histological slide.
Figure 3.
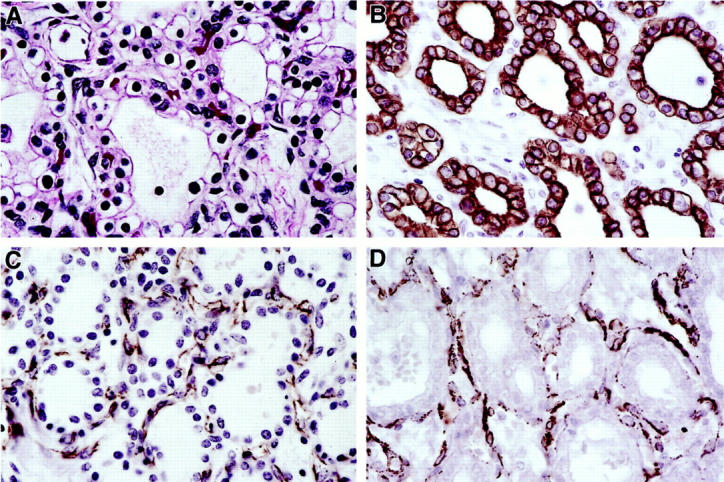
H&E and immunohistochemical stains in a VHL pancreatic MCA. A: Epithelial and endothelial cells (H&E). B: Positive cytokeratin MAK6 stain in the epithelial cells. C: Positive CD31 stain in the endothelial cells. D: Positive SMA stain in the smooth muscle cells. (all ×630).
DNA Extraction
Procured cells were resuspended immediately in 10 to 20 μl of buffer containing Tris/HCl, pH 8.0, 10 mmol/L ethylenediamine tetra-acetic acid, pH 8.0, 1% Tween 20, and 0.1 mg/ml proteinase K, and were incubated at 37°C overnight. The mixture was boiled for 10 minutes to inactivate protinase K, and 1.5 μl of this solution was used for the polymerase chain reaction (PCR) amplification of DNA.
LOH Analysis
Twenty-seven cystic lesions from three informative patients were analyzed for LOH with two microsatellite markers, D3S1110 and D3S1038 (Research Genetics, Huntsville, AL), flanking the VHL gene on chromosome 3p25.5. 17,25 For both microsatellite markers PCR was performed for 35 cycles: denaturing at 95°C for 1 minute, annealing at 55°C for 40 seconds, and extending at 72°C for 1 minute. For all primers, the final extension was continued for 10 minutes.
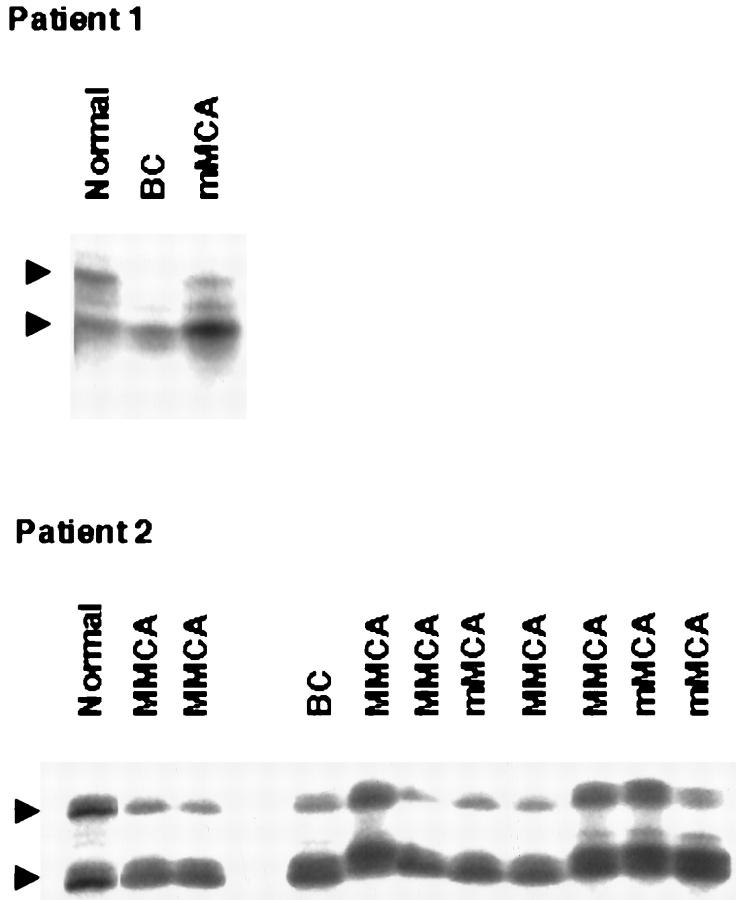
Each PCR sample contained 1.5 μl of template DNA as noted above, 10 pmol of each primer, 20 nmol each of dATP, dCTP, DGTP, and DTTP, 15 mmol/L MgCl2, 0.1 U of Taq DNA polymerase, 0.05 μl of [32P] dCTP (6000 Ci/mmol), and 1 μl of 10× buffer in a total volume of 10 μl. Labeled amplified DNA was mixed with an equal volume of formamide loading dye (95% formamide, 20 mmol/L EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol) and analyzed on a single-strand conformation polymorphism gel. 22 The samples were denatured for 5 minutes at 95°C and loaded onto a gel consisting of 6% acrylamide (49:1 acrylamide:bis), 5% glycerol, and 0.6× TBE. Samples were electrophoresed at 8W at room temperature overnight. Gels were transferred to 3-mm Whatman paper and dried, and autoradiography was performed with Kodak X-OMAT film (Eastman Kodak, Rochester, NY). The complete absence or 70% decreased intensity of one allele on acrylamide gel was interpreted as LOH. Seventy percent decrease in intensity of the wild-type allele was used for LOH scoring in this study because contaminating endothelial cells could not be completely excluded during microdissection of the epithelial cells (Figure 4) ▶ . Each result was reproduced 2–3 times.
Figure 4.
Representative results of LOH analysis at the VHL gene with a polymorphic marker D3S1110 in patients 1 and 2. Arrowheads show positions of both alleles. In patient 1, the upper allele is deleted in both BC and mMCA. In patient 2, the upper allele is deleted in 4 out of 6 MMCA, 2 out of 3 mMCA, and a BC as compared to normal pancreatic tissue. Endothelial cells that are closely intermixed with epithelial cells in pancreatic serous lesions are likely to contribute to contamination in LOH analysis data.
Results
In all nine VHL patients, pancreatic cystic lesions were multiple. The lesions >0.4 cm in size were seen grossly as cysts, and, in cases of MCA, as a conglomerate of cysts ranging in size from 0.5 to 18 cm or subtotally replacing normal pancreatic parenchyma (Figure 2) ▶ . We classified the lesions as follows: 21 BC in 5 patients (average number per patient 2.1; range, 0–8), 35 MMCA in 9 patients (average number per patient 3.0; range, 0–21), and 63 mMCA in 9 patients (average number per patient 7.7; range, 1–37) (Table 1) ▶ . Histologically, MCA contained prominent fibrous stroma, and all lesions were surrounded by fibrous tissue (Figures 1 and 2) ▶ ▶ . Occasionally entrapped islets of Langerhans were seen in fibrous stroma. All lesions were cystic and, rarely, solid in architecture. A predominant population of bland, cuboidal, and flattened serous epithelial cells with clear and/or amphophilic cytoplasm, lack of nuclear atypia, mitoses, or necrosis was present in all lesions. The cells were strongly positive for cytokeratins AE1/AE3 and MAK 6, and rich in intracytoplasmic glycogen on PAS/PAS-D stains (Figures 2 and 3) ▶ ▶ . The second population of numerous endothelial cells forming capillaries and closely intermixed with epithelial cells was consistently seen on H&E and CD31 stains in all BC, mMCA, and MMCA in the study (Figures 1 and 3) ▶ ▶ . Furthermore, immunohistochemistry revealed the third cell type in all lesions: a smooth muscle cell, which was strongly positive for vimentin and SMA (Figure 3) ▶ . Electron microscopy performed on five representative lesions confirmed the presence of the above three cell types and didn’t demonstrate dense core endocrine granules. Surrounding pancreas was normal or, in cases of large MCA, showed compression of the exocrine and endocrine tissue.
Figure 2.
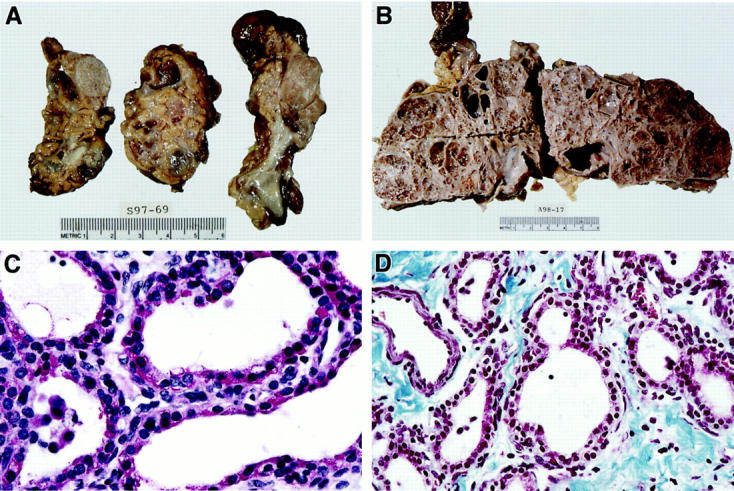
Gross pathology and histochemistry of VHL pancreatic cystic lesions. A: Multifocal cystic and solid pancreatic lesions. B: Subtotal replacement of pancreatic parenchyma by cystic disease. C: Prominent glycogen in the epithelial cells (PAS stain) (×630). D: Prominent stromal fibrosis (Masson’s trichrome stain) (×400).
DNA from 27 microdissected BC, mMCA, MMCA, and normal exocrine and endocrine pancreas was analyzed for LOH at the VHL gene locus using polymorphic markers D3S1110 and D3S1038 in three patients with known germline mutations (Table 1 ▶ ; patients 1, 2, and 4). These patients were informative for both markers; ie, their normal DNA showed two different alleles (heterozygosity). In three patients analyzed, LOH for both markers was detected in 24 out of 27 different cystic lesions (Figure 4) ▶ . LOH of the same wild-type allele was consistently seen in multiple lesions from an individual patient. Failure to detect LOH in two MMCA and one mMCA of patient 2 (Figure 4) ▶ could be due to contaminating endothelial cells.
Discussion
This report is the first detailed morphological and molecular genetic description of pancreatic cystic disease in VHL patients. We identified a spectrum of multiple pancreatic cystic lesions located throughout the pancreas and subdivided them by size and architecture into BC, mMCA, and MMCA. Our data demonstrate that all three subtypes of cystic lesions represent architectural and histological variants of the same molecular genetic process rather than three separate entities. Histologically, all lesions demonstrated a mixture of clear and/or amphophilic, glycogen-rich epithelial cells, endothelial cells, and smooth muscle cells, and showed prominent fibrosis. On the molecular level, the epithelial cells in 24 different lesions exhibited loss of the wild-type allele at the VHL gene locus, providing evidence of the neoplastic nature and close relationship of pancreatic serous cysts and MCA and establishing them as an integral part of VHL disease.
Clinically, pancreatic cystic lesions in VHL are benign and follow an indolent course. In cases with large MCA, biliary obstruction, pain from bowel compression, and exocrine or endocrine insufficiency have been observed. However, the vast majority of VHL patients with MCA remains asymptomatic, shows preservation of normal exocrine and endocrine pancreatic function, and doesn’t require surgery for many years despite radiological evidence of extensive pancreatic cystic disease.
Genotype/phenotype correlation has been reported in VHL disease. VHL type 1 phenotype (without pheochromocytoma) and VHL type 2 phenotype (with pheochromocytoma) have been described. 28 The most frequent VHL mutation hot spot at the nucleotide 712/713 predisposes carriers to a VHL type 2 phenotype with a high risk of pheochromocytoma and RCC development. 29 Concurrent central nervous system hemangioblastoma was present in all nine patients in the study (Table 1) ▶ . Six patients had a concurrent retinal angioma, and six patients had a pheochromocytoma. Four of the nine patients had a concurrent pancreatic neuroendocrine tumor (NET). Eight VHL patients with pancreatic cystic disease had a germline mutation or partial deletion in exons 1 or 3 of the VHL gene. Four of the mutations resulted in frame shift and protein truncation, and five were missense mutations (Table 1) ▶ . We did not observe a phenotype/genotype correlation in this study of nine VHL patients in whom pancreatic cystic tumors were discovered incidentally. A larger VHL population study of patients with pancreatic cystic disease would be necessary to investigate a phenotype/genotype correlation question further.
Differential diagnosis of pancreatic cystic neoplasia includes primary sporadic neoplasms of the pancreas such as mucinous cystadenoma, mucinous cystadenocarcinoma, adenocarcinoma, sugar tumor, and NET. 30-33 Importantly, in VHL patients many lesions, ie, hemangioblastoma, retinal angioma, cystadenoma of the epidydimis, pancreatic NET, and RCC, demonstrate clear cell cytology and numerous small vessels. It is the histopathological similarity with both pancreatic NET and RCC metastases that may lead to misdiagnosis and, subsequently, to erroneous treatment of VHL patients. Both MCA and pancreatic NET contain cells that are cytokeratin-positive, demonstrate cytoplasmic glycogen on PAS stain, and lack acidic mucin on mucicarmine stain. However, unlike NET, MCA is negative for S-100 and chromogranin A and lacks dense core granules and lipid globules on electron microscopic examination. 3 MCA is a benign neoplasm in asymptomatic patients; pancreatic NET has a malignant potential to metastasize to the liver when it is 2 to 3 cm in size, and requires surgical excision. 4
Histologically, pancreatic cysts and MCA are strikingly similar to renal cysts and clear cell RCC in VHL patients. 20 Both pancreatic and renal lesions are cystic or solid in architecture, contain cytokeratin MAK6-positive, glycogen- rich epithelial cells, and have prominent small vessels. Unlike pancreatic MCA, clear cell RCC in VHL contains cytoplasmic lipids and demonstrates numerous red blood cells in cystic spaces. Renal cysts and RCC show fibrous pseudocapsule and may have stromal fibrosis. However, prominent stromal fibrosis and the presence of vimentin/SMA-positive smooth muscle cells are important features of pancreatic BC and MCA. Unlike benign MCA, VHL RCC has a known malignant potential. 34 Although metastases of clear cell RCC to the pancreas have been reported in sporadic RCC cases, they are rare. 35,36 In the setting of VHL disease, awareness of histopathological similarities between pancreatic MCA, NET, and RCC 3,20 as well as other VHL lesions, and communication between a pathologist and clinician are of the utmost importance for the follow-up, prognosis, and treatment of patients.
In summary, our data provide morphological and molecular genetic evidence that VHL pancreatic cystic disease is a neoplastic process in that BC and mMCA arise at multiple foci in the pancreas, grow, and coalesce, forming MMCA. Because multiple BC and MCA of the pancreas are caused by alteration of the VHL gene, they represent serous cyst-MCA continuum in the development of pancreatic serous neoplasia and should be considered an integral part of VHL disease.
Footnotes
Address reprint requests to Irina A. Lubensky, M.D., Molecular Pathogenesis Unit, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, NIH, Building 10, Room 5D37, 10 Center Drive, Bethesda, MD 20892. E-mail: lubenskyi@ninds.nih.gov.
References
- 1.Lamiell JM, Salazar FG, Hsia YE: Von Hippel-Lindau disease affecting 43 members of a single kindred. Medicine (Baltimore) 1989, 68:1-29 [DOI] [PubMed] [Google Scholar]
- 2.Binkovitz LA, Johnson CD, Stephens DH: Islet cell tumors in von Hippel-Lindau disease: increased prevalence and relationship to the multiple endocrine neoplasias. Am J Roentgenol 1990, 155:501-505 [DOI] [PubMed] [Google Scholar]
- 3.Lubensky IA, Pack S, Ault A, Vortmeyer AO, Libutti SK, Choyke PL, Walther MM, Linehan MW, Zhuang Z: Multiple neuroendocrine tumors of the pancreas in von Hippel-Lindau disease patients: histopathological and molecular genetic analysis. Am J Pathol 1998, 153:223-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libutti SK, Choyke PL, Bartlett DL, Vargas HA, Walther M, Lubensky I, Glenn G, Steinberg S, Linehan WM, Alexander HR: Pancreatic neuroendocrine tumors associated with von Hippel-Lindau disease: diagnostic and management recommendations. Surgery 1998, 124:1153-1159 [DOI] [PubMed] [Google Scholar]
- 5.Neumann HPH, Dinkel E, Brambs H, Wimmer B, Friedburg H, Volk B, Sigmund G, Riegler P, Haag K, Schollmeyer P, Wiestler OD: Pancreatic lesions in the von Hippel-Lindau syndrome. Gastoenterology 1991, 101:465-471 [DOI] [PubMed] [Google Scholar]
- 6.Hough MT, Stephens DH, Johnson CD, Binkovitz LA: Pancreatic lesions in von Hippel-Lindau disease: prevalence, clinical significance, and CT findings. Am J Roentgenol 1994, 162:1091-1094 [DOI] [PubMed] [Google Scholar]
- 7.Compagno J, Oertel JE: Microcystic adenomas of the pancreas (glycogen-rich cystadenomas). Am J Clin Pathol 1976, 69:289-298 [DOI] [PubMed] [Google Scholar]
- 8.Alpert LC, Truong LD, Bossart MI, Spjut HJ: Microcystic adenoma (serous cystadenoma) of the pancreas. Am J Surg Pathol 1988, 12:251-263 [DOI] [PubMed] [Google Scholar]
- 9.Shorten SD, Hart WR, Petras RE: Microcystic adenomas (serous cystadenomas) of pancreas. Am J Surg Pathol 1986, 10:365-372 [DOI] [PubMed] [Google Scholar]
- 10.Nyongo A, Huntrakoon M: Microcystic adenoma of the pancreas with myoepithelial cells. Am J Clin Pathol 1985, 84:114-120 [DOI] [PubMed] [Google Scholar]
- 11.Morohoshi T, Held G, Klöppel G: Exocrine pancreatic tumours and their histological classification: a study based on 167 autopsy and 97 surgical cases. Histopathology 1983, 7:645-661 [DOI] [PubMed] [Google Scholar]
- 12.Beerman MH, Fromkes JJ, Carey LC, Thomas FB: Pancreatic cystadenoma in von Hippel-Lindau disease: an unusual cause of pancreatic and common bile duct obstruction. J Clin Gastroenterol 1982, 4:537-540 [DOI] [PubMed] [Google Scholar]
- 13.Bickler S, Wile AG, Melicharek M, Recher L: Pancreatic involvement in Hippel-Lindau disease. West J Med 1984, 140:280-282 [PMC free article] [PubMed] [Google Scholar]
- 14.Horton WA, Wong V, Eldbidge R: Von Hippel-Lindau disease: clinical and pathological manifestations in nine families with 50 affected members. Arch Intern Med 1976, 136:769-777 [DOI] [PubMed] [Google Scholar]
- 15.Latif F, Tory K, Gnarra J, Yao M, Duh F-M, Orcutt M, Stackhouse T, Kuzmin I, Modi W, Geil L, Schmidt L, Zhou F, Li H, Wei M, Chen F, Glenn G, Choyke P, Walther M, Weng Y, Duan D, Dean M, Glavac D, Richards F, Crossey P, Ferguson-Smith M, Paslier D, Chumakov I, Cohen D, Chinault A, Maher E, Linehan W, Zbar B, Lerman M: Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 1993, 260:1317-1320 [DOI] [PubMed] [Google Scholar]
- 16.Knudson AG: Hereditary cancer, oncogenes, and antioncogenes. Cancer Res 1985, 45:1437-1443 [PubMed] [Google Scholar]
- 17.Vortmeyer AO, Gnarra JR, Emmert-Buck MR, Katz D, Linehan WM, Oldfield EH, Zhuang Z: VHL gene deletion detected in the stromal cell component of a cerebellar hemangioblastoma associated with von Hippel-Lindau disease. Hum Pathol 1997, 28:540-543 [DOI] [PubMed] [Google Scholar]
- 18.Tse JYM, Wong JHC, Lo K, Poon W, Huang DP, Ng H: Molecular genetic analysis of the von Hippel-Lindau disease suppressor gene in familial and sporadic cerebellar hemangioblastomas. Am J Clin Pathol 1997, 107:459-466 [DOI] [PubMed] [Google Scholar]
- 19.Chan C, Vortmeyer AO, Chew EY, Green R, Matteson D, Shen D, Linehan WM, Lubensky IA, Zhuang Z: VHL gene deletion and enhanced VEGF gene expression detected in the stromal cells of retinal angioma. Arch Ophthalmol 1999, 117:625-630 [DOI] [PubMed] [Google Scholar]
- 20.Lubensky IA, Gnarra J, Bertheau P, Walther M, Linehan WM, Zhuang Z: Allelic deletions of the VHL gene detected in multiple microscopic clear cell renal lesions in von Hippel-Lindau disease patients: Am J Pathol 1996, 149:2089-2094 [PMC free article] [PubMed] [Google Scholar]
- 21.Crossey PA, Foster K, Richards FM, Phipps ME, Latif F, Tory K, Jones MH, Bentley E, Kumar R, Lerman MI, Zbar B, Affara NA, Ferguson-Smith MA, Maher ER: Molecular geneic investigations of the mechanism of tumourigenesis in von Hippel-lindau disease: analysis of allele loss in VHL tumours. Hum Genet 1994, 93:53-58 [DOI] [PubMed] [Google Scholar]
- 22.Zeiger M, Zbar B, Keiser H, Linehan WM, Gnarra JR: Loss of heterozygosity on the short arm of chromosome 3 in sporadic, von Hippel-Lindau disease-associated, and familial pheochromocytoma. Genes Chromosomes Cancer 1995, 13:151-156 [DOI] [PubMed] [Google Scholar]
- 23.Tory K, Brauch H, Linehan WM, Barba D, Oldfield E, Filling-Katz M, Seizinger B, Nakamura Y, White R, Marshall FF, Lerman MI, Zbar B: Specific genetic change in tumors associated with von Hippel-Lindau disease. J Natl Cancer Inst 1989, 81:1097-1101 [DOI] [PubMed] [Google Scholar]
- 24.Vortmeyer AO, Choo D, Pack SD, Oldfield E, Zhuang Z: von Hippel-Lindau disease gene alterations associated with endolymphatic sac tumor (letter). J Natl Cancer Inst 1997, 89:970-972 [DOI] [PubMed] [Google Scholar]
- 25.Vortmeyer AO, Lubensky IA, Fogt F, Linehan WM, Khettry U, Zhuang Z: Allelic deletion and mutation of the VHL tumor suppressor gene in pancreatic microcystic adenomas. Am J Pathol 1997, 151:951-956 [PMC free article] [PubMed] [Google Scholar]
- 26.Choyke PL, Glenn GM, Walther MM, Patronas NJ, Linehan WM, Zbar B: von Hippel-Lindau disease: genetic, clinical, and imaging features. Radiology 1995, 194:629-642 [DOI] [PubMed] [Google Scholar]
- 27.Zhuang Z, Bertheau P, Emmert-Buck M, Liotta L, Gnarra J, Linehan W, Lubensky I: A microdissection technique for archival DNA analysis of specific cell populations in lesions <1 mm in size. Am J Pathol 1995, 146:620-625 [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann HPH, Wiestler OD: Clustering of features of von Hippel-Lindau disease: evidence of complex genetic locus. Lancet 1991, 337:1052-1054 [DOI] [PubMed] [Google Scholar]
- 29.Chen F, Kishida T, Masahiro Y, Hustad T, Glavac D, Dean M, Gnarra J, Orcutt M, Duh F, Glenn G, Green J, Hsia Y, Lamiell J, Li H, Wei M, Schmidt L, Tory K, Kuzmin I, Stackhouse T, Latif F, Linehan WM, Lerman M, Zbar B: Germline mutations in the von Hippel-Lindau disease tumor suppressor gene: correlation with phenotype. Human Mutat 1995, 5:66-75 [DOI] [PubMed] [Google Scholar]
- 30.Perez-Ordonez B, Naseem A, Lieberman PH, Klimstra DS: Solid serous adenoma of the pancreas: the solid variant of serous cystadenoma? Am J Surg Pathol 1996, 20:1401-1405 [DOI] [PubMed] [Google Scholar]
- 31.Solcia E, Capella C, Klöppel G: Tumors of the exocrine pancreas. Tumors of the Pancreas. Atlas of Tumor Pathology. Edited by Rosai J. Washington, DC, Armed Forces Institute of Pathology, 1997, pp 31–144
- 32.Zamboni G, Pea M, Martignoni G, Zancanaro C, Faccioli G, Gilioli E, Pederzoli P, Bonetti F: Clear cell “sugar” tumor of the pancreas: a novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol 1996, 20:722-730 [DOI] [PubMed] [Google Scholar]
- 33.Solcia E, Capella C, Klöppel G: Tumors of the endocrine pancreas. Tumors of the Pancreas. Atlas of Tumor Pathology. Edited by Rosai J. Washington, DC, Armed Forces Institute of Pathology, 1997, pp 145–209
- 34.Walther M, Choyke P, Weiss G, Manolatos C, Long J, Reiter R, Linehan W: Parenchymal sparing surgery in patients with hereditary renal cell carcinoma. J Urol 1995, 153:913-916 [PubMed] [Google Scholar]
- 35.Strijk SP: Pancreatic metastases of renal cell carcinoma: report of two cases. Gastrointest Radiol 1989, 14:123-126 [DOI] [PubMed] [Google Scholar]
- 36.Temellini F, Bavosi M, Lamarra M, Quagliarini P, Giuliani F: Pancreatic metastasis 25 years after nephrectomy for renal cancer. Tumori 1989, 75:503-504 [DOI] [PubMed] [Google Scholar]