Figure 5.
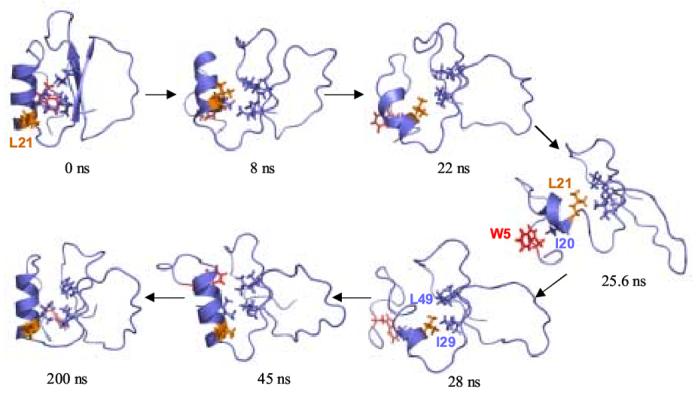
Hydrophobic core interactions in the unfolding and refolding pathways. The side chains of residues Trp 5, Ile 20, Leu 21, Ile 29, Val 31, Val 47, and Leu 49 are shown. Trp 5 is colored red and Leu 21 is orange. As the protein unfolds, the α-helix twists and Leu 21 is buried in the core of the protein and the α-helix unfolds. In the most distorted structure, at 25.6 ns, the protein is held together by a nonnative cluster consisting of Leu 21, Ile 29, Ile 30, and Leu 49. The α-helix twists back as it refolds, and Ile 20 reforms its native contacts in the core.
