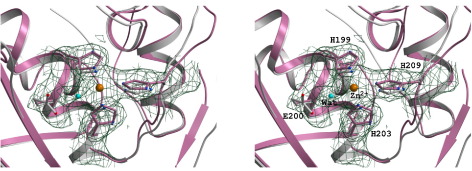
Figure 2.
Structure of the active site of the E200A variant of human MMP-1 superimposed with the wild-type enzyme (PDB code: 1CGL).20 The mutant enzyme is in pink and the wild-type enzyme in grey. The water molecule at the catalytic site that is important for peptide bond hydrolysis is shown in cyan and the catalytic zinc is displayed in orange. Electron density (2Fo-Fc map contoured at 1.0σ) is shown around the catalytic site residues, the catalytic zinc and the water molecule.