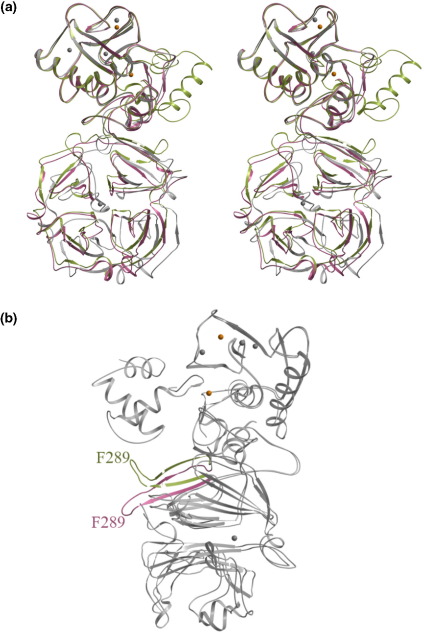
Figure 5.
Conformational differences in the hemopexin-like domain of proMMP-1 and active MMP-1. (a) Shown in pink is human active MMP-1, in grey is porcine MMP-1 and green is for human proMMP-1. Superposition was based on the catalytic domain of all the three molecules. The stereo view highlights the relative movement of the hemopexin-like domain of proMMP-1 and active MMP-1. Activation of the pro-enzyme (green: closed configuration) results in an open-conformation (pink/grey: active MMP-1). (b) Major displacement of the Phe289-Tyr290-Pro291 loop of the hemopexin-like domain in the pro and active form of the human enzyme. Both forms of the enzyme have been coloured grey except for the displaced loop, which is shown in pink in the active enzyme and in green in the pro-enzyme. All the Pigures were made using the program MOLSCRIPT.30