Abstract
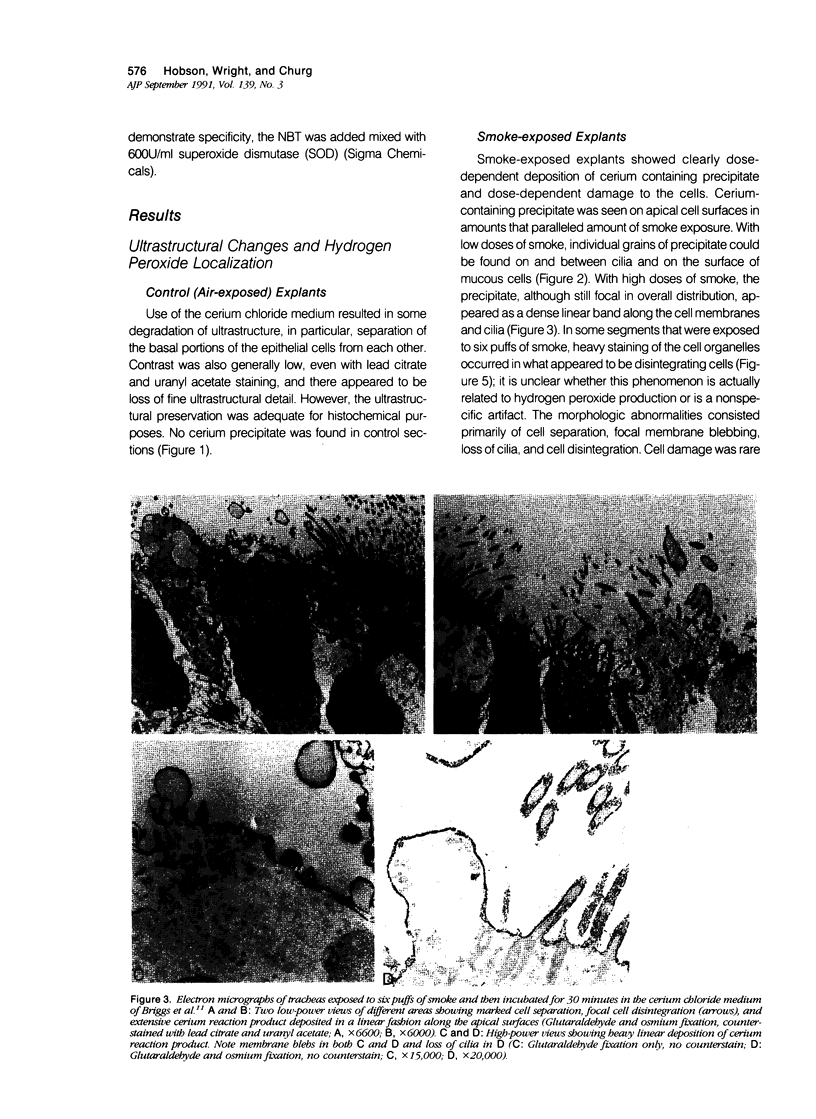
Cigarette smoke is known to contain many types of free radicals, and solutions of smoke tar have been shown to liberate hydrogen peroxide as well as superoxide radical. To further investigate the relationship of smoke exposure and generation of active oxygen species, the authors exposed rat tracheal explants to varying amounts of smoke for 10 minutes in a humidified chamber. After smoke exposure was completed, tracheal segments were incubated in a modification of the ultrastructural cerium chloride technique that was devised by Briggs et al. to demonstrate hydrogen peroxide production. Smoke dose-dependent deposition of cerium-containing reaction product was found on the cilia and the apical membranes; with low-dose smoke, the reaction product appeared as individual dots along the apical surface, but with greater amounts of smoke, heavy linear deposits of reaction product were found along the apical membranes. Smoke produced focal dose-related cell damage with blebbing of the apical membranes, loss of cilia, and focal cell necrosis. Catalase prevented both the positive histochemical reaction and the cell damage; if the catalase was first boiled, its protective effect was destroyed. Similarly, after smoke exposure was completed, tracheal segments were covered with a solution of nitroblue tetrazolium to demonstrate production of superoxide anion. A positive reaction was observed by light microscopy on the surface of tracheas that was exposed to smoke but not that exposed to air; the reaction could be prevented by addition of superoxide dismutase. The authors conclude that exposure of tracheal explants to cigarette smoke in vitro is associated with histochemical evidence of continuing production of both hydrogen peroxide and superoxide anion at the apical cell membrane.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burman W. J., Martin W. J., 2nd Oxidant-mediated ciliary dysfunction. Possible role in airway disease. Chest. 1986 Mar;89(3):410–413. doi: 10.1378/chest.89.3.410. [DOI] [PubMed] [Google Scholar]
- Church D. F., Pryor W. A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985 Dec;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churg A., Hobson J., Berean K., Wright J. Scavengers of active oxygen species prevent cigarette smoke-induced asbestos fiber penetration in rat tracheal explants. Am J Pathol. 1989 Oct;135(4):599–603. [PMC free article] [PubMed] [Google Scholar]
- Churg A., Hobson J., Wright J. Effects of cigarette smoke dose and time after smoke exposure on uptake of asbestos fibers by rat tracheal epithelial cells. Am J Respir Cell Mol Biol. 1990 Sep;3(3):265–269. doi: 10.1165/ajrcmb/3.3.265. [DOI] [PubMed] [Google Scholar]
- Cosgrove J. P., Borish E. T., Church D. F., Pryor W. A. The metal-mediated formation of hydroxyl radical by aqueous extracts of cigarette tar. Biochem Biophys Res Commun. 1985 Oct 15;132(1):390–396. doi: 10.1016/0006-291x(85)91034-4. [DOI] [PubMed] [Google Scholar]
- Goodglick L. A., Kane A. B. Cytotoxicity of long and short crocidolite asbestos fibers in vitro and in vivo. Cancer Res. 1990 Aug 15;50(16):5153–5163. [PubMed] [Google Scholar]
- Goodglick L. A., Kane A. B. Role of reactive oxygen metabolites in crocidolite asbestos toxicity to mouse macrophages. Cancer Res. 1986 Nov;46(11):5558–5566. [PubMed] [Google Scholar]
- Goodglick L. A., Pietras L. A., Kane A. B. Evaluation of the causal relationship between crocidolite asbestos-induced lipid peroxidation and toxicity to macrophages. Am Rev Respir Dis. 1989 May;139(5):1265–1273. doi: 10.1164/ajrccm/139.5.1265. [DOI] [PubMed] [Google Scholar]
- Hinshaw D. B., Sklar L. A., Bohl B., Schraufstatter I. U., Hyslop P. A., Rossi M. W., Spragg R. G., Cochrane C. G. Cytoskeletal and morphologic impact of cellular oxidant injury. Am J Pathol. 1986 Jun;123(3):454–464. [PMC free article] [PubMed] [Google Scholar]
- Hobson J., Gilks B., Wright J., Churg A. Direct enhancement by cigarette smoke of asbestos fiber penetration and asbestos-induced epithelial proliferation in rat tracheal explants. J Natl Cancer Inst. 1988 Jun 1;80(7):518–521. doi: 10.1093/jnci/80.7.518. [DOI] [PubMed] [Google Scholar]
- Hobson J., Wright J. L., Churg A. Active oxygen species mediate asbestos fiber uptake by tracheal epithelial cells. FASEB J. 1990 Oct;4(13):3135–3139. doi: 10.1096/fasebj.4.13.2170219. [DOI] [PubMed] [Google Scholar]
- Mossman B. T., Craighead J. E. Use of hamster tracheal organ cultures for assessing the cocarcinogenic effects of inorganic particulates on the respiratory epithelium. Prog Exp Tumor Res. 1979;24:37–47. doi: 10.1159/000402082. [DOI] [PubMed] [Google Scholar]
- Mossman B. T., Marsh J. P. Evidence supporting a role for active oxygen species in asbestos-induced toxicity and lung disease. Environ Health Perspect. 1989 May;81:91–94. doi: 10.1289/ehp.898191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Church D. F., Pryor W. A. Quantitative analysis of the hydrogen peroxide formed in aqueous cigarette tar extracts. Free Radic Biol Med. 1989;7(1):9–15. doi: 10.1016/0891-5849(89)90094-4. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Kodama M., Nagata C. Generation of hydrogen peroxide and superoxide anion radical from cigarette smoke. Gan. 1984 Feb;75(2):95–98. [PubMed] [Google Scholar]
- Nikula K. J., Wilson D. W., Giri S. N., Plopper C. G., Dungworth D. L. The response of the rat tracheal epithelium to ozone exposure. Injury, adaptation, and repair. Am J Pathol. 1988 May;131(2):373–384. [PMC free article] [PubMed] [Google Scholar]
- Raghu G., Striker L., Harlan J., Gown A., Striker G. Cytoskeletal changes as an early event in hydrogen peroxide-induced cell injury: a study in A549 cells. Br J Exp Pathol. 1986 Feb;67(1):105–112. [PMC free article] [PubMed] [Google Scholar]