Abstract
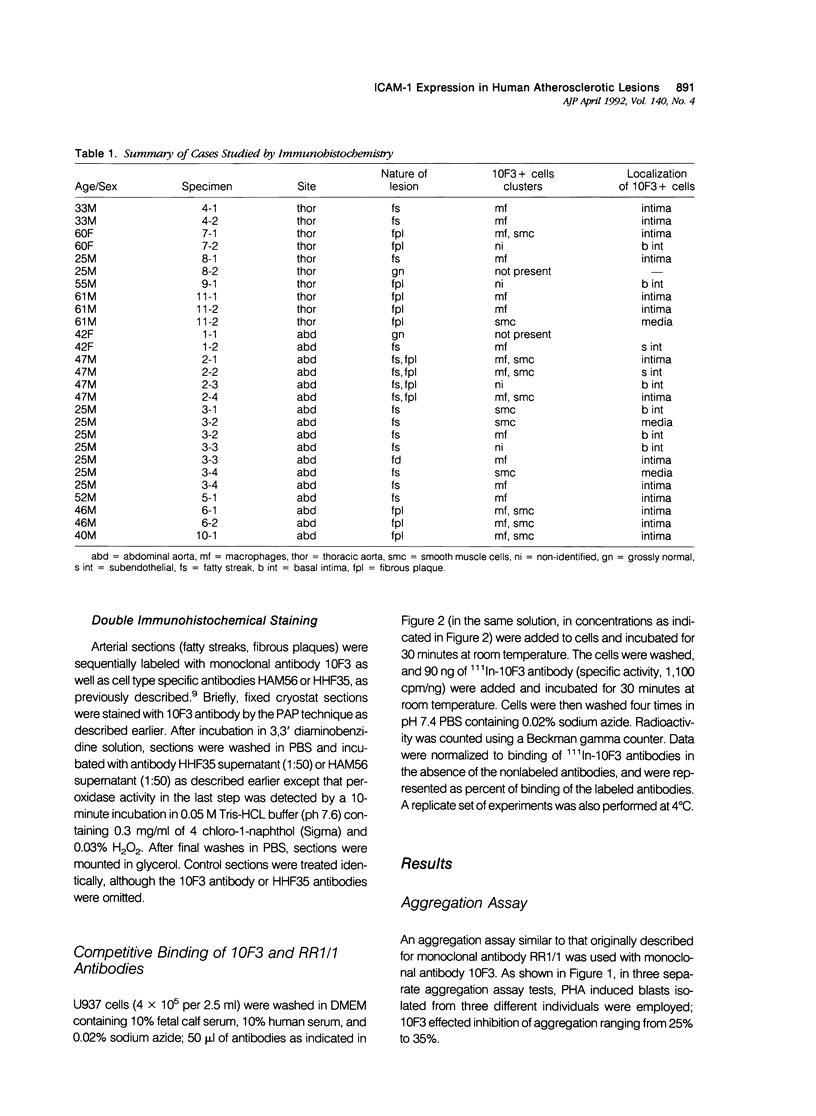
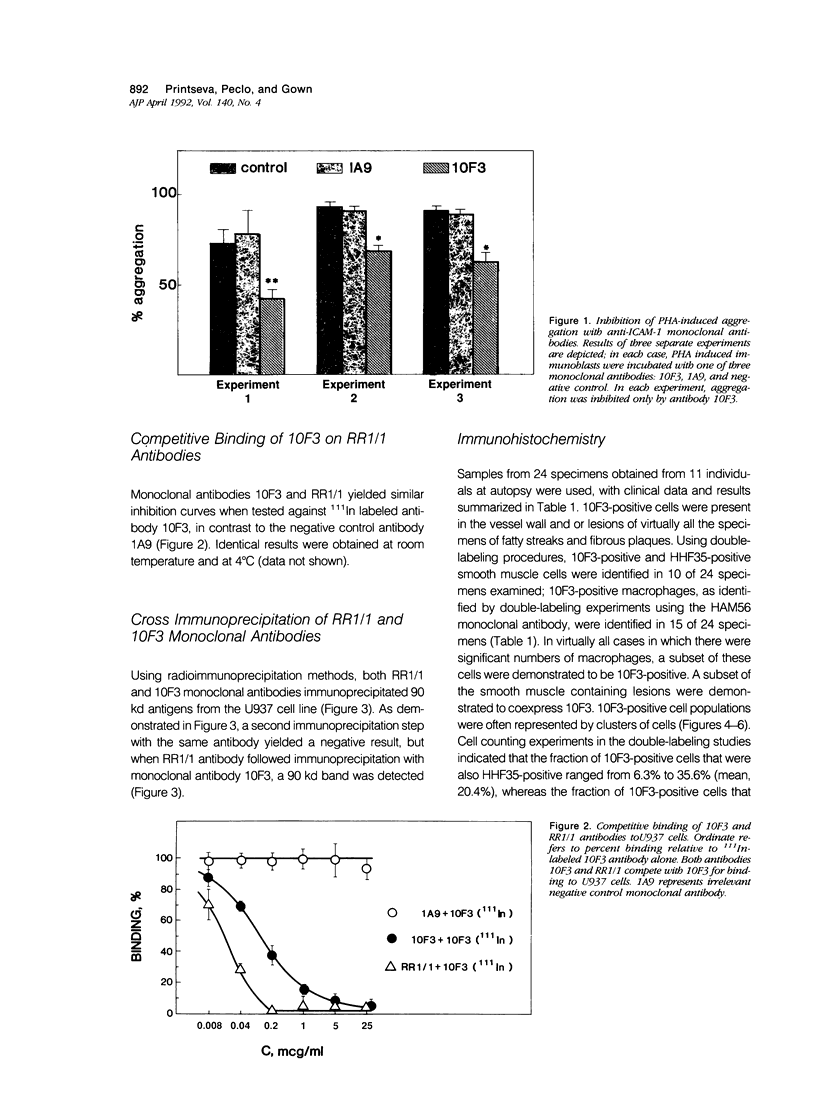
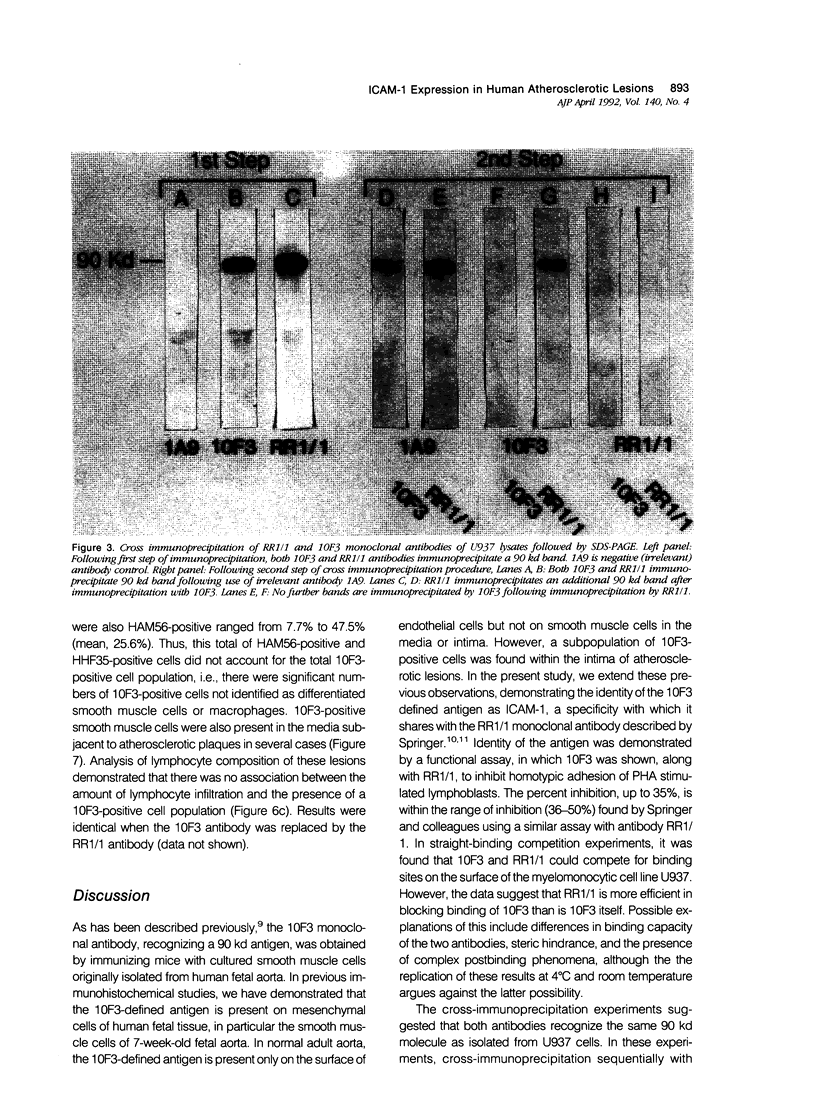
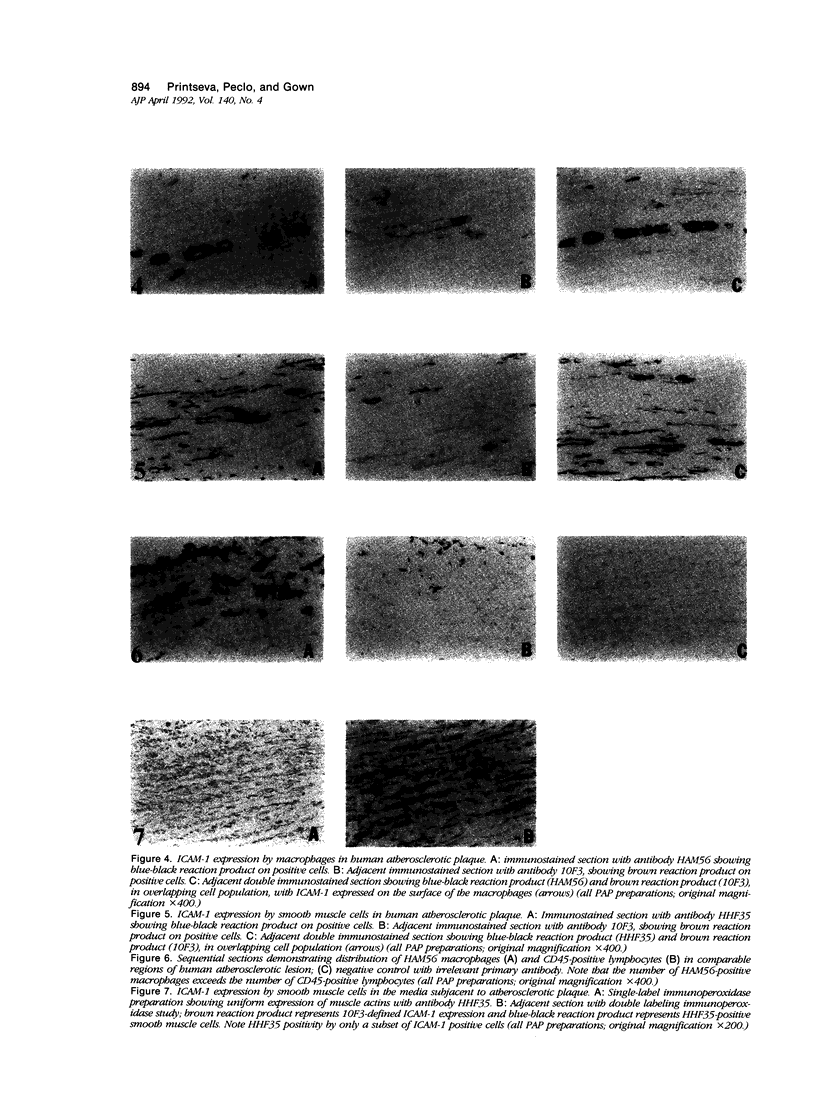
The specificity of monoclonal antibody 10F3, generated to smooth muscle cells isolated from fetal human aorta, has been further explored in a series of biological, biochemical, and immunocytochemical studies. In the first assay, it was found that 10F3 could inhibit aggregation of phytohemagglutinin (PHA)-induced lymphocytes in a manner comparable to that of antibody RR1/1, an anti-intercellular adhesion molecule 1 (ICAM-1) monoclonal antibody. In immunoprecipitation experiments followed by one-dimensional gel electrophoresis, both 10F3 and RR1/1 immunoprecipitated 90 kd proteins, with results suggesting that the two antibodies recognized different epitopes of the same molecule. A series of immunocytochemical studies on human atherosclerotic lesions was performed; using single-labeling techniques, 10F3-positive cells were found in the vessel wall and in lesions of virtually all specimens of fatty streaks and fibrous plaques. Using double-labeling techniques, 10F3-positive macrophages and 10F3-positive smooth muscle cells were found; however, there were also a significant number of non-smooth muscle, nonmacrophage 10F3-positive cells. These studies demonstrate that 10F3 identifies ICAM-1, and that this protein is expressed on a variety of cell types in human atherosclerotic lesions. ICAM-1 may represent a developmentally regulated protein that is expressed in fetal but not adult mesenchymal cells, but can be re-expressed in pathologic processes such as atherosclerosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood C. H., Zetter B. R. Tumor interactions with the vasculature: angiogenesis and tumor metastasis. Biochim Biophys Acta. 1990 Jun 1;1032(1):89–118. doi: 10.1016/0304-419x(90)90014-r. [DOI] [PubMed] [Google Scholar]
- Campbell J. H., Kocher O., Skalli O., Gabbiani G., Campbell G. R. Cytodifferentiation and expression of alpha-smooth muscle actin mRNA and protein during primary culture of aortic smooth muscle cells. Correlation with cell density and proliferative state. Arteriosclerosis. 1989 Sep-Oct;9(5):633–643. doi: 10.1161/01.atv.9.5.633. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Faerman A. I., Chervonskii A. V., Chipysheva T. A., Gel'shtein V. I., Koliada A. Iu. Poluchenie monoklonal'nykh antitel k peroksidaze khrena i ikh primenenie v immunogistokhimii i immunoblottinge. Biull Eksp Biol Med. 1987 May;103(5):631–634. [PubMed] [Google Scholar]
- Folkman J. What is the role of angiogenesis in metastasis from cutaneous melanoma? Eur J Cancer Clin Oncol. 1987 Apr;23(4):361–363. doi: 10.1016/0277-5379(87)90370-1. [DOI] [PubMed] [Google Scholar]
- Geer J. C. Fine structure of human aortic intimal thickening and fatty streaks. Lab Invest. 1965 Oct;14(10):1764–1783. [PubMed] [Google Scholar]
- Glukhova M. A., Kabakov A. E., Frid M. G., Ornatsky O. I., Belkin A. M., Mukhin D. N., Orekhov A. N., Koteliansky V. E., Smirnov V. N. Modulation of human aorta smooth muscle cell phenotype: a study of muscle-specific variants of vinculin, caldesmon, and actin expression. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9542–9546. doi: 10.1073/pnas.85.24.9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Johnson J. P., Stade B. G., Holzmann B., Schwäble W., Riethmüller G. De novo expression of intercellular-adhesion molecule 1 in melanoma correlates with increased risk of metastasis. Proc Natl Acad Sci U S A. 1989 Jan;86(2):641–644. doi: 10.1073/pnas.86.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Kocher O., Gabbiani G. Cytoskeletal features of normal and atheromatous human arterial smooth muscle cells. Hum Pathol. 1986 Sep;17(9):875–880. doi: 10.1016/s0046-8177(86)80637-2. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Cordell J. L., Abdulaziz Z., Naiem M., Bordenave G. Preparation of peroxidase: antiperoxidase (PAP) complexes for immunohistological labeling of monoclonal antibodies. J Histochem Cytochem. 1982 Nov;30(11):1114–1122. doi: 10.1177/30.11.6183312. [DOI] [PubMed] [Google Scholar]
- Mosse P. R., Campbell G. R., Campbell J. H. Smooth muscle phenotypic expression in human carotid arteries. II. Atherosclerosis-free diffuse intimal thickenings compared with the media. Arteriosclerosis. 1986 Nov-Dec;6(6):664–669. doi: 10.1161/01.atv.6.6.664. [DOI] [PubMed] [Google Scholar]
- Mosse P. R., Campbell G. R., Wang Z. L., Campbell J. H. Smooth muscle phenotypic expression in human carotid arteries. I. Comparison of cells from diffuse intimal thickenings adjacent to atheromatous plaques with those of the media. Lab Invest. 1985 Nov;53(5):556–562. [PubMed] [Google Scholar]
- Printseva O. J., Peclo M. M., Tjurmin A. V., Faerman A. I., Danilov S. M., Repin V. S., Smirnov V. N. A 90-kd surface antigen from a subpopulation of smooth muscle cells from human atherosclerotic lesions. Am J Pathol. 1989 Feb;134(2):305–313. [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Schmidt R. A., Gown A. M. "Professional" and "nonprofessional" contractile cells in the lung. Am J Respir Cell Mol Biol. 1990 Dec;3(6):513–514. doi: 10.1165/ajrcmb/3.6.513. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Thompson W. S., Miller L. J., Schmalstieg F. C., Anderson D. C. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J Exp Med. 1984 Dec 1;160(6):1901–1918. doi: 10.1084/jem.160.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Warner S. J., Libby P. Human vascular smooth muscle cells. Target for and source of tumor necrosis factor. J Immunol. 1989 Jan 1;142(1):100–109. [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Williams L. T., Schwartz S. M., Gordon D. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest. 1988 Sep;82(3):1134–1143. doi: 10.1172/JCI113671. [DOI] [PMC free article] [PubMed] [Google Scholar]







