Abstract
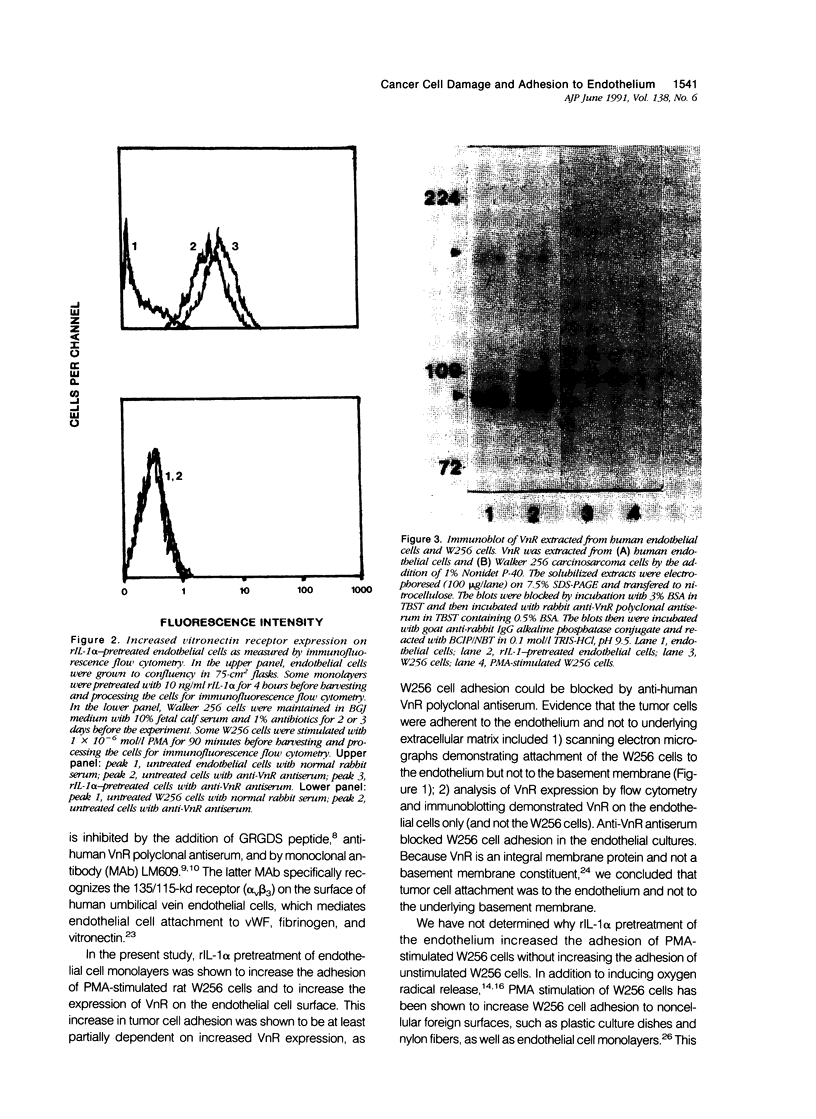
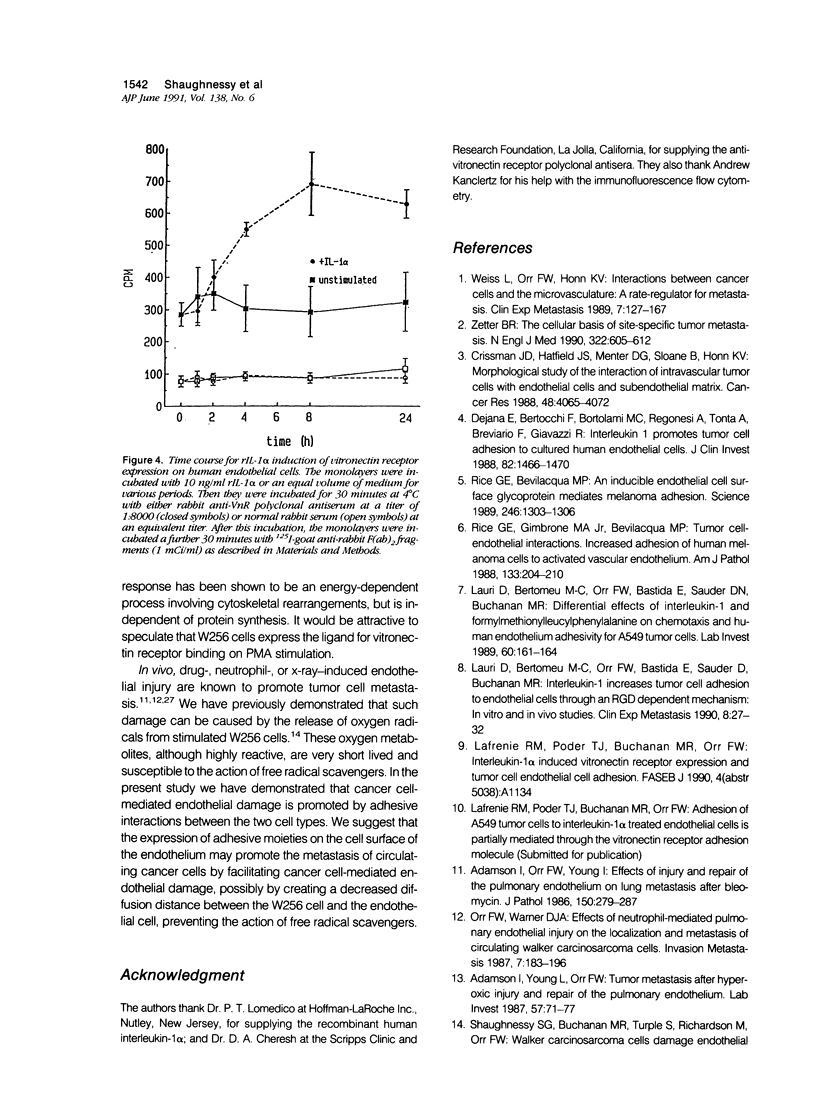
The transport of cancer cells from blood vessels to extravascular tissue is a critical step in metastasis, where endothelial cells and the vascular basement membrane act as barriers to cell traffic. Because endothelial injury can facilitate the metastasis of intravascular cancer cells in vivo, the authors have studied in vitro the free-radical-mediated endothelial damage caused by the rat Walker 256 carcinosarcoma (W256) cell after stimulation with 10(-6) mol/l (molar) phorbol ester. Here the authors have examined the hypothesis that W256 cell-mediated endothelial injury is dependent on adhesion between the effector and target cells. Attachment of phorbol 12-myristate, 13-acetate (PMA)-stimulated W256 cells to endothelial monolayers was increased 1.8 +/- 0.1-fold and damage (3H-2-deoxyglucose release from labeled endothelium) 1.4 +/- 0.1-fold after 4-hour pretreatment of the endothelium with 10 ng/ml recombinant human interleukin-1 alpha (rIL-1 alpha). Under various assay conditions, the release of 3H-2-deoxyglucose correlated directly with tumor cell adhesion (r = 0.98, P less than 0.005). In the presence of a polyclonal anti-vitronectin receptor antiserum, adhesion of stimulated W256 cells to rIL-1 alpha-treated monolayers was inhibited by 39% +/- 2%, and 3H-2-deoxyglucose release was inhibited by 53% +/- 13%. Immunoblot analysis and immunofluorescence flow cytometry demonstrated that the endothelial cells but not the W256 cells expressed vitronectin receptor (VnR) on their cell surface. The surface expression of VnR by endothelial cells was increased 1.9 +/- 0.1-fold after 4 hours' incubation with rIL-1 alpha. The authors conclude that W256 cell-mediated endothelial damage is dependent on cell adhesion, which, in turn, is partly regulated by the expression of VnR on the endothelial cell surface.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Orr F. W., Young L. Effects of injury and repair of the pulmonary endothelium on lung metastasis after bleomycin. J Pathol. 1986 Dec;150(4):279–287. doi: 10.1002/path.1711500407. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Young L., Orr F. W. Tumor metastasis after hyperoxic injury and repair of the pulmonary endothelium. Lab Invest. 1987 Jul;57(1):71–77. [PubMed] [Google Scholar]
- Andreoli S. P., Baehner R. L., Bergstein J. M. In vitro detection of endothelial cell damage using 2-deoxy-D-3H-glucose: comparison with chromium 51, 3H-leucine, 3H-adenine, and lactate dehydrogenase. J Lab Clin Med. 1985 Sep;106(3):253–261. [PubMed] [Google Scholar]
- Auerbach R., Lu W. C., Pardon E., Gumkowski F., Kaminska G., Kaminski M. Specificity of adhesion between murine tumor cells and capillary endothelium: an in vitro correlate of preferential metastasis in vivo. Cancer Res. 1987 Mar 15;47(6):1492–1496. [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Harper J. R. Arg-Gly-Asp recognition by a cell adhesion receptor requires its 130-kDa alpha subunit. J Biol Chem. 1987 Feb 5;262(4):1434–1437. [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crissman J. D., Hatfield J. S., Menter D. G., Sloane B., Honn K. V. Morphological study of the interaction of intravascular tumor cells with endothelial cells and subendothelial matrix. Cancer Res. 1988 Jul 15;48(14):4065–4072. [PubMed] [Google Scholar]
- Dejana E., Bertocchi F., Bortolami M. C., Regonesi A., Tonta A., Breviario F., Giavazzi R. Interleukin 1 promotes tumor cell adhesion to cultured human endothelial cells. J Clin Invest. 1988 Oct;82(4):1466–1470. doi: 10.1172/JCI113753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Hirata H., Tanaka K. Artificial metastases and decrease of fibrinolysis in the nude mouse lung after hemithoracic irradiation. Clin Exp Metastasis. 1984 Oct-Dec;2(4):311–319. doi: 10.1007/BF00135170. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lauri D., Bertomeu M. C., Orr F. W., Bastida E., Sauder D. N., Buchanan M. R. Differential effects of interleukin-1 and formylmethionylleucylphenylalanine on chemotaxis and human endothelium adhesivity for A549 tumor cells. Lab Invest. 1989 Jan;60(1):161–164. [PubMed] [Google Scholar]
- Lauri D., Bertomeu M. C., Orr F. W., Bastida E., Sauder D., Buchanan M. R. Interleukin-1 increases tumor cell adhesion to endothelial cells through an RGD dependent mechanism: in vitro and in vivo studies. Clin Exp Metastasis. 1990 Jan-Feb;8(1):27–32. doi: 10.1007/BF00155590. [DOI] [PubMed] [Google Scholar]
- Leroyer V., Werner L., Shaughnessy S., Goddard G. J., Orr F. W. Chemiluminescence and oxygen radical generation by Walker carcinosarcoma cells following chemotactic stimulation. Cancer Res. 1987 Sep 15;47(18):4771–4775. [PubMed] [Google Scholar]
- Orr F. W., Warner D. J. Effects of neutrophil-mediated pulmonary endothelial injury on the localization and metastasis of circulating Walker carcinosarcoma cells. Invasion Metastasis. 1987;7(3):183–196. [PubMed] [Google Scholar]
- Rice G. E., Bevilacqua M. P. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989 Dec 8;246(4935):1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- Rice G. E., Gimbrone M. A., Jr, Bevilacqua M. P. Tumor cell-endothelial interactions. Increased adhesion of human melanoma cells to activated vascular endothelium. Am J Pathol. 1988 Nov;133(2):204–210. [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Argraves W. S., Pytela R., Arai H., Krusius T., Pierschbacher M. D., Ruoslahti E. cDNA and amino acid sequences of the cell adhesion protein receptor recognizing vitronectin reveal a transmembrane domain and homologies with other adhesion protein receptors. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8614–8618. doi: 10.1073/pnas.83.22.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J., Fantone J. C. Phorbol myristate acetate-induced adherence of Walker 256 carcinosarcoma cells. Cancer Res. 1982 Jan;42(1):190–197. [PubMed] [Google Scholar]
- Weiss L., Orr F. W., Honn K. V. Interactions between cancer cells and the microvasculature: a rate-regulator for metastasis. Clin Exp Metastasis. 1989 Mar-Apr;7(2):127–167. doi: 10.1007/BF01787020. [DOI] [PubMed] [Google Scholar]
- Zetter B. R. The cellular basis of site-specific tumor metastasis. N Engl J Med. 1990 Mar 1;322(9):605–612. doi: 10.1056/NEJM199003013220907. [DOI] [PubMed] [Google Scholar]