Abstract
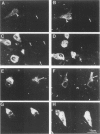
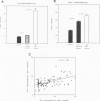
Previous studies have shown altered casein kinase II (CK-II) in Alzheimer's disease (AD). For the present study, the authors analyzed CK-II immunoreactivity at various stages of tangle formation using quantitative laser confocal microscopy and immunoelectron microscopy. AD hippocampal pyramidal cells without neurofibrillary tangles (NFTs) displayed 15% more anti-tau immunoreactivity (P less than 0.01) and 43% more anti-CKII immunolabeling than controls (P less than 0.001). In AD, tangle-bearing hippocampal neurons with strong anti-tau immunoreactivity (threefold increase from controls) showed a significant 22% increase in anti-CKII immunolabeling (P less than 0.01), compared with those without NFTs. Neurons with early neurofibrillary changes showed diffuse anti-CKII immunostaining in their cytoplasm and cell processes. In tangle-bearing neurons, in which a higher level of tau immunoreactivity was detected, anti-CKII immunolabeling was distributed along a fibrillar meshwork in cell bodies and processes. Linear regression analysis of anti-CKII and anti-tau immunoreactivity in AD showed a positive correlation (r = 0.53, P less than 0.001). At the ultrastructural level, anti-CKII was immunolocalized to the paired helical filaments (PHF) of the tangle-bearing neurons, as well as to PHF in neuropil threads and some dystrophic neurites in plaques. These results suggest a possible role for CK-II in tangle formation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksenova M. V., Burbaeva G. S., Kandror K. V., Kapkov D. V., Stepanov A. S. The decreased level of casein kinase 2 in brain cortex of schizophrenic and Alzheimer's disease patients. FEBS Lett. 1991 Feb 11;279(1):55–57. doi: 10.1016/0014-5793(91)80249-3. [DOI] [PubMed] [Google Scholar]
- Apel E. D., Litchfield D. W., Clark R. H., Krebs E. G., Storm D. R. Phosphorylation of neuromodulin (GAP-43) by casein kinase II. Identification of phosphorylation sites and regulation by calmodulin. J Biol Chem. 1991 Jun 5;266(16):10544–10551. [PubMed] [Google Scholar]
- Arendt T., Taubert G., Bigl V., Arendt A. Amyloid deposition in the nucleus basalis of Meynert complex: a topographic marker for degenerating cell clusters in Alzheimer's disease. Acta Neuropathol. 1988;75(3):226–232. doi: 10.1007/BF00690530. [DOI] [PubMed] [Google Scholar]
- Bancher C., Brunner C., Lassmann H., Budka H., Jellinger K., Wiche G., Seitelberger F., Grundke-Iqbal I., Iqbal K., Wisniewski H. M. Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res. 1989 Jan 16;477(1-2):90–99. doi: 10.1016/0006-8993(89)91396-6. [DOI] [PubMed] [Google Scholar]
- Baum L., Masliah E., Iimoto D. S., Hansen L. A., Halliday W. C., Saitoh T. Casein kinase II is associated with neurofibrillary tangles but is not an intrinsic component of paired helical filaments. Brain Res. 1992 Feb 21;573(1):126–132. doi: 10.1016/0006-8993(92)90121-o. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Cole G. M., Timiras P. S. Ubiquitin-protein conjugates in Alzheimer's lesions. Neurosci Lett. 1987 Aug 18;79(1-2):207–212. doi: 10.1016/0304-3940(87)90698-7. [DOI] [PubMed] [Google Scholar]
- Dahmus M. E. Purification and properties of calf thymus casein kinases I and II. J Biol Chem. 1981 Apr 10;256(7):3319–3325. [PubMed] [Google Scholar]
- Filhol O., Cochet C., Chambaz E. M. DNA binding activity of casein kinase II. Biochem Biophys Res Commun. 1990 Dec 31;173(3):862–871. doi: 10.1016/s0006-291x(05)80866-6. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986 May 5;261(13):6084–6089. [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamos J. E., DeGennaro L. J., Drachman D. A. Synaptic loss in Alzheimer's disease and other dementias. Neurology. 1989 Mar;39(3):355–361. doi: 10.1212/wnl.39.3.355. [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Arai T., Ihara Y. Immunochemical evidence that fragments of phosphorylated MAP5 (MAP1B) are bound to neurofibrillary tangles in Alzheimer's disease. Neuron. 1990 Jun;4(6):909–918. doi: 10.1016/0896-6273(90)90144-5. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinases--multipotential protein kinases. Curr Top Cell Regul. 1982;21:101–127. [PubMed] [Google Scholar]
- Ihara Y., Nukina N., Miura R., Ogawara M. Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer's disease. J Biochem. 1986 Jun;99(6):1807–1810. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- Iimoto D. S., Masliah E., DeTeresa R., Terry R. D., Saitoh T. Aberrant casein kinase II in Alzheimer's disease. Brain Res. 1990 Jan 22;507(2):273–280. doi: 10.1016/0006-8993(90)90282-g. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Morris J. H., Selkoe D. J., Kosik K. S. Tau epitopes are incorporated into a range of lesions in Alzheimer's disease. J Neuropathol Exp Neurol. 1987 Nov;46(6):611–622. doi: 10.1097/00005072-198711000-00001. [DOI] [PubMed] [Google Scholar]
- KIDD M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963 Jan 12;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Duffy L. K., Dowling M. M., Abraham C., McCluskey A., Selkoe D. J. Microtubule-associated protein 2: monoclonal antibodies demonstrate the selective incorporation of certain epitopes into Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7941–7945. doi: 10.1073/pnas.81.24.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht S. A., Schwartz B., Avigdor A., Guberman R. Growth-related enzyme activities in crypt compartments during rat colon carcinogenesis. Anticancer Res. 1990 May-Jun;10(3):773–778. [PubMed] [Google Scholar]
- Masliah E., Hansen L., Albright T., Mallory M., Terry R. D. Immunoelectron microscopic study of synaptic pathology in Alzheimer's disease. Acta Neuropathol. 1991;81(4):428–433. doi: 10.1007/BF00293464. [DOI] [PubMed] [Google Scholar]
- Masliah E., Mallory M., Hansen L., Alford M., Albright T., DeTeresa R., Terry R., Baudier J., Saitoh T. Patterns of aberrant sprouting in Alzheimer's disease. Neuron. 1991 May;6(5):729–739. doi: 10.1016/0896-6273(91)90170-5. [DOI] [PubMed] [Google Scholar]
- Masliah E., Terry R. D., Alford M., DeTeresa R., Hansen L. A. Cortical and subcortical patterns of synaptophysinlike immunoreactivity in Alzheimer's disease. Am J Pathol. 1991 Jan;138(1):235–246. [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Terry R. D., Mallory M., Alford M., Hansen L. A. Diffuse plaques do not accentuate synapse loss in Alzheimer's disease. Am J Pathol. 1990 Dec;137(6):1293–1297. [PMC free article] [PubMed] [Google Scholar]
- Mujumdar R. B., Ernst L. A., Mujumdar S. R., Waggoner A. S. Cyanine dye labeling reagents containing isothiocyanate groups. Cytometry. 1989 Jan;10(1):11–19. doi: 10.1002/cyto.990100104. [DOI] [PubMed] [Google Scholar]
- Münstermann U., Fritz G., Seitz G., Lu Y. P., Schneider H. R., Issinger O. G. Casein kinase II is elevated in solid human tumours and rapidly proliferating non-neoplastic tissue. Eur J Biochem. 1990 Apr 30;189(2):251–257. doi: 10.1111/j.1432-1033.1990.tb15484.x. [DOI] [PubMed] [Google Scholar]
- Perry G., Friedman R., Shaw G., Chau V. Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci U S A. 1987 May;84(9):3033–3036. doi: 10.1073/pnas.84.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. L., Davis P. B., Morris J. C., White D. L. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging. 1991 Jul-Aug;12(4):295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Masliah E., Jin L. W., Cole G. M., Wieloch T., Shapiro I. P. Protein kinases and phosphorylation in neurologic disorders and cell death. Lab Invest. 1991 May;64(5):596–616. [PubMed] [Google Scholar]
- Steiner B., Mandelkow E. M., Biernat J., Gustke N., Meyer H. E., Schmidt B., Mieskes G., Söling H. D., Drechsel D., Kirschner M. W. Phosphorylation of microtubule-associated protein tau: identification of the site for Ca2(+)-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J. 1990 Nov;9(11):3539–3544. doi: 10.1002/j.1460-2075.1990.tb07563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERRY R. D., GONATAS N. K., WEISS M. ULTRASTRUCTURAL STUDIES IN ALZHEIMER'S PRESENILE DEMENTIA. Am J Pathol. 1964 Feb;44:269–297. [PMC free article] [PubMed] [Google Scholar]
- Terry R. D., Peck A., DeTeresa R., Schechter R., Horoupian D. S. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981 Aug;10(2):184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Nakazato Y., Hirai S., Shoji M. Immunoelectron microscopic localization of amyloid beta protein in the diffuse plaques of Alzheimer-type dementia. Brain Res. 1990 Feb 5;508(2):320–324. doi: 10.1016/0006-8993(90)90416-9. [DOI] [PubMed] [Google Scholar]