Abstract
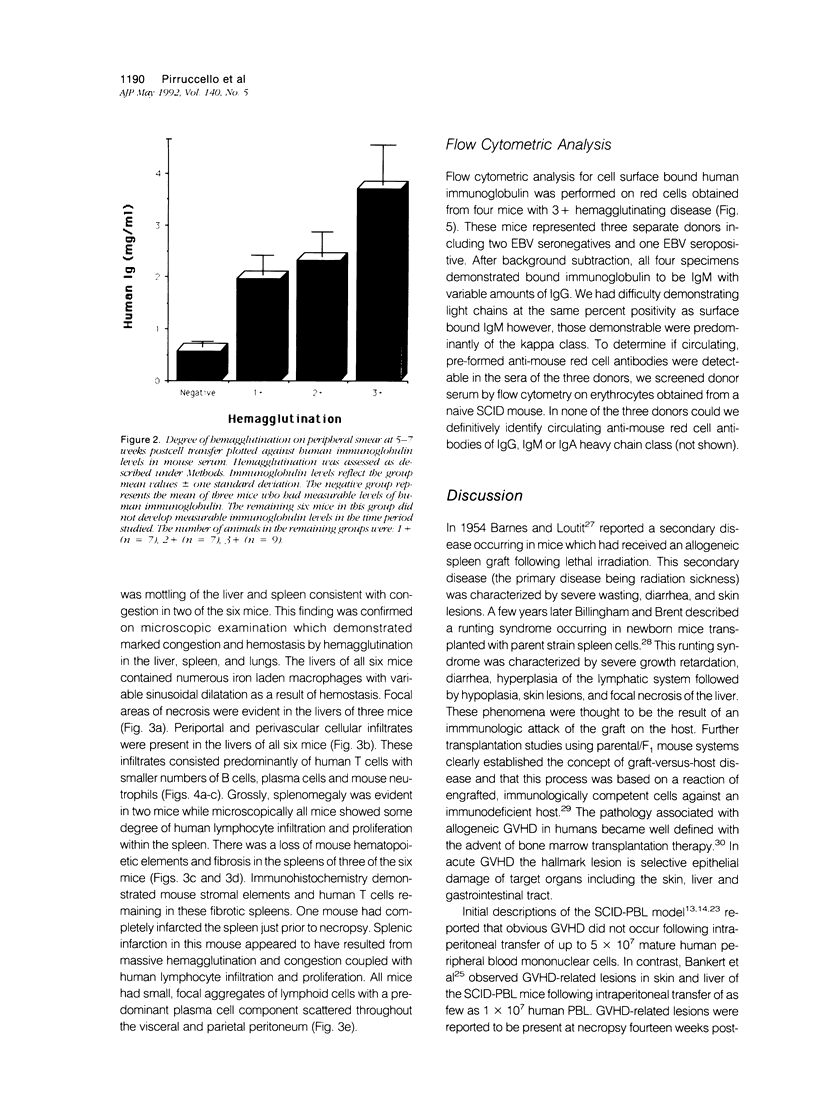
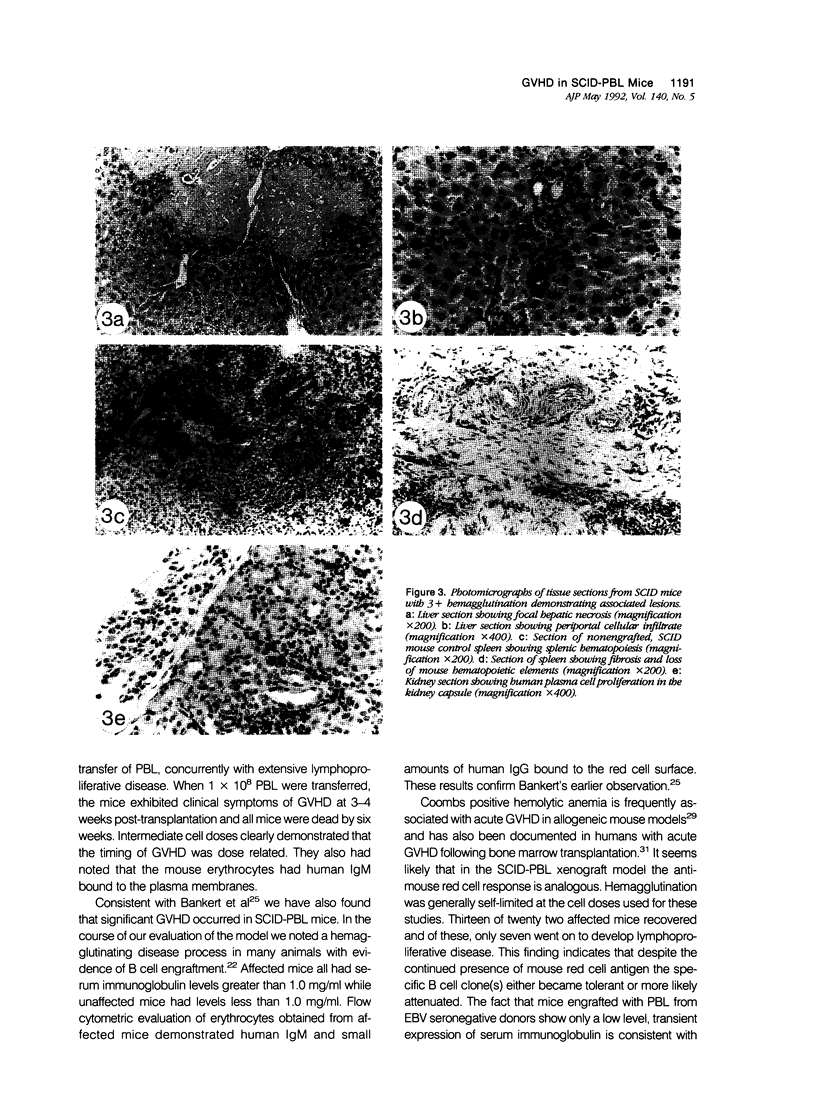
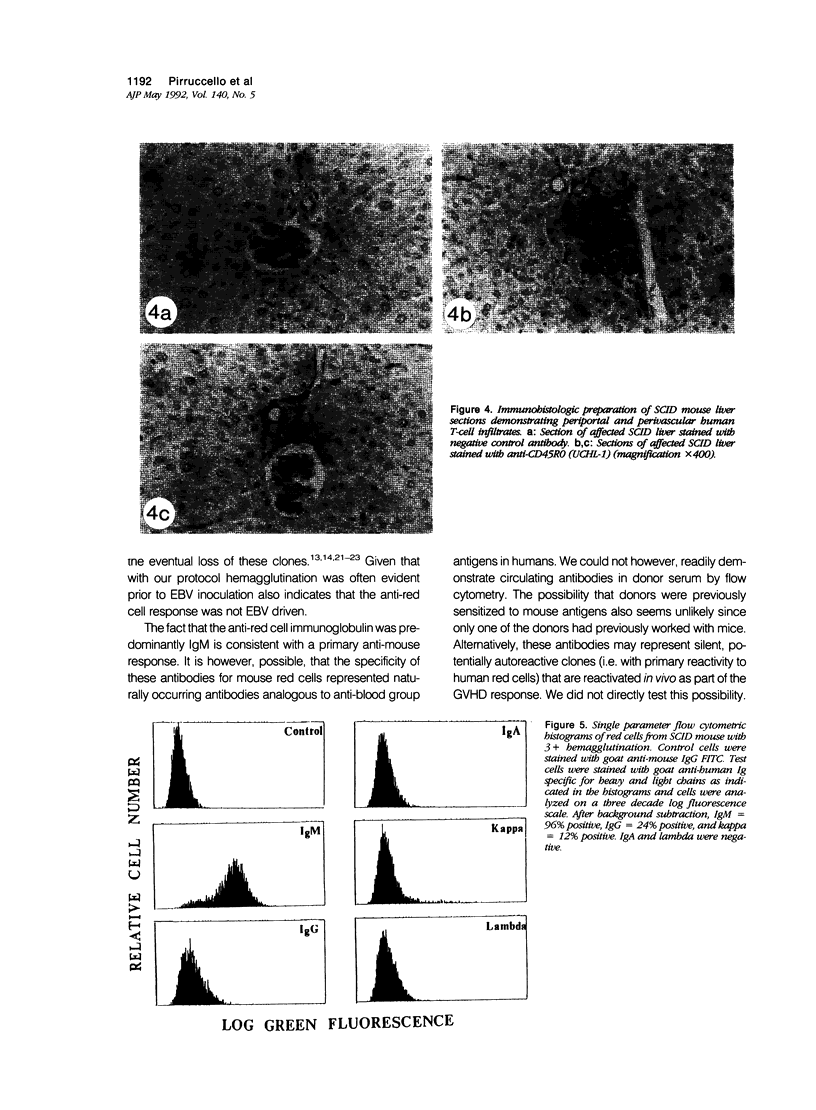
In the course of evaluating the severe combined immunodeficiency mouse-human peripheral blood lymphocyte (SCID-PBL) model of lymphoproliferative disease, we noted hemagglutination occurring in peripheral blood smears of mice with serum human immunoglobulin levels greater than 1.0 mg/ml. The hemagglutinating process was mediated by human anti-mouse red cell antibodies of the IgM class, peaked at five to seven weeks post-transfer of 5 to 7 x 10(7) human PBL and was generally self limiting. However, death resulted in some mice when serum immunoglobulin levels were greater than 3.0 mg/ml. The most severely affected mice had hemagglutination induced congestion of liver, lungs and spleen. Several mice also had lesions consistent with graft-versus-host disease (GVHD) including focal hepatic necrosis and destruction of mouse splenic hematopoietic elements. The lesions associated with hemagglutination and GVHD in SCID-PBL mice are distinct from those associated with EBV-induced lymphoproliferation. Recognition of these pathologic processes are required for a thorough understanding of the SCID-PBL model.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLINGHAM R. E., BRENT L. A simple method for inducing tolerance of skin homografts in mice. Transplant Bull. 1957 Apr;4(2):67–71. [PubMed] [Google Scholar]
- Bankert R. B., Umemoto T., Sugiyama Y., Chen F. A., Repasky E., Yokota S. Human lung tumors, patients' peripheral blood lymphocytes and tumor infiltrating lymphocytes propagated in scid mice. Curr Top Microbiol Immunol. 1989;152:201–210. doi: 10.1007/978-3-642-74974-2_24. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Cannon M. J., Pisa P., Fox R. I., Cooper N. R. Epstein-Barr virus induces aggressive lymphoproliferative disorders of human B cell origin in SCID/hu chimeric mice. J Clin Invest. 1990 Apr;85(4):1333–1337. doi: 10.1172/JCI114573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. M., Hardy R. R., Petrini J., Bosma M. J. T cell leakiness in scid mice. Curr Top Microbiol Immunol. 1989;152:117–123. doi: 10.1007/978-3-642-74974-2_15. [DOI] [PubMed] [Google Scholar]
- Custer R. P., Bosma G. C., Bosma M. J. Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am J Pathol. 1985 Sep;120(3):464–477. [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K., Keller G. M., Phillips R. A., Miller R. G., Bosma G. C., O'Toole M., Bosma M. J. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984 Apr;132(4):1804–1808. [PubMed] [Google Scholar]
- Dorshkind K., Pollack S. B., Bosma M. J., Phillips R. A. Natural killer (NK) cells are present in mice with severe combined immunodeficiency (scid). J Immunol. 1985 Jun;134(6):3798–3801. [PubMed] [Google Scholar]
- Duchosal M. A., McConahey P. J., Robinson C. A., Dixon F. J. Transfer of human systemic lupus erythematosus in severe combined immunodeficient (SCID) mice. J Exp Med. 1990 Sep 1;172(3):985–988. doi: 10.1084/jem.172.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara J. L., Deeg H. J. Graft-versus-host disease. N Engl J Med. 1991 Mar 7;324(10):667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- Fulop G. M., Phillips R. A. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990 Oct 4;347(6292):479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- Gatti R. A., Meuwissen H. J., Allen H. D., Hong R., Good R. A. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968 Dec 28;2(7583):1366–1369. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- Ghetie M. A., Richardson J., Tucker T., Jones D., Uhr J. W., Vitetta E. S. Disseminated or localized growth of a human B-cell tumor (Daudi) in SCID mice. Int J Cancer. 1990 Mar 15;45(3):481–485. doi: 10.1002/ijc.2910450318. [DOI] [PubMed] [Google Scholar]
- Hendrickson E. A., Schlissel M. S., Weaver D. T. Wild-type V(D)J recombination in scid pre-B cells. Mol Cell Biol. 1990 Oct;10(10):5397–5407. doi: 10.1128/mcb.10.10.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kamel-Reid S., Letarte M., Sirard C., Doedens M., Grunberger T., Fulop G., Freedman M. H., Phillips R. A., Dick J. E. A model of human acute lymphoblastic leukemia in immune-deficient SCID mice. Science. 1989 Dec 22;246(4937):1597–1600. doi: 10.1126/science.2595371. [DOI] [PubMed] [Google Scholar]
- Kim M. G., Schuler W., Bosma M. J., Marcu K. B. Abnormal recombination of Igh D and J gene segments in transformed pre-B cells of scid mice. J Immunol. 1988 Aug 15;141(4):1341–1347. [PubMed] [Google Scholar]
- Malynn B. A., Blackwell T. K., Fulop G. M., Rathbun G. A., Furley A. J., Ferrier P., Heinke L. B., Phillips R. A., Yancopoulos G. D., Alt F. W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988 Aug 12;54(4):453–460. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Kaneshima H., Shultz L. D., Lieberman M., Weissman I. L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988 Sep 23;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Shih C. C., Rabin L., Kaneshima H. Suppression of HIV infection in AZT-treated SCID-hu mice. Science. 1990 Feb 2;247(4942):564–566. doi: 10.1126/science.2300816. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Spector S., Spector D., Kipps T. J., Fox R. I., Carson D. A., Cooper N., Richman D. D. Studies of HIV infection and the development of Epstein-Barr virus-related B cell lymphomas following transfer of human lymphocytes to mice with severe combined immunodeficiency. Curr Top Microbiol Immunol. 1989;152:195–199. doi: 10.1007/978-3-642-74974-2_23. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B., Spector D. H., Spector S. A. Human immunodeficiency virus infection of human-PBL-SCID mice. Science. 1991 Feb 15;251(4995):791–794. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988 Sep 15;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Nakamine H., Yokote H., Itakura T., Hayashi S., Komai N., Takano Y., Saito K., Moriwaki H., Nishino E., Takenaka T. Non-Hodgkin's lymphoma involving the brain. Diagnostic usefulness of stereotactic needle biopsy in combination with paraffin-section immunohistochemistry. Acta Neuropathol. 1989;78(5):462–471. doi: 10.1007/BF00687707. [DOI] [PubMed] [Google Scholar]
- Namikawa R., Kaneshima H., Lieberman M., Weissman I. L., McCune J. M. Infection of the SCID-hu mouse by HIV-1. Science. 1988 Dec 23;242(4886):1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- Okano M., Taguchi Y., Nakamine H., Pirruccello S. J., Davis J. R., Beisel K. W., Kleveland K. L., Sanger W. G., Fordyce R. R., Purtilo D. T. Characterization of Epstein-Barr virus-induced lymphoproliferation derived from human peripheral blood mononuclear cells transferred to severe combined immunodeficient mice. Am J Pathol. 1990 Sep;137(3):517–522. [PMC free article] [PubMed] [Google Scholar]
- Petrini J. H., Carroll A. M., Bosma M. J. T-cell receptor gene rearrangements in functional T-cell clones from severe combined immune deficient (scid) mice: reversion of the scid phenotype in individual lymphocyte progenitors. Proc Natl Acad Sci U S A. 1990 May;87(9):3450–3453. doi: 10.1073/pnas.87.9.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirruccello S. J., Bicak M. S., Gordon B. G., Gajl-Peczalska K., Gnarra D. J., Coccia P. F. Acute lymphoblastic leukemia of NK-cell lineage: responses to IL-2. Leuk Res. 1989;13(9):735–743. doi: 10.1016/0145-2126(89)90086-6. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T., Falk K., Pirruccello S. J., Nakamine H., Kleveland K., Davis J. R., Okano M., Taguchi Y., Sanger W. G., Beisel K. W. SCID mouse model of Epstein-Barr virus-induced lymphomagenesis of immunodeficient humans. Int J Cancer. 1991 Feb 20;47(4):510–517. doi: 10.1002/ijc.2910470407. [DOI] [PubMed] [Google Scholar]
- Rowe M., Young L. S., Crocker J., Stokes H., Henderson S., Rickinson A. B. Epstein-Barr virus (EBV)-associated lymphoproliferative disease in the SCID mouse model: implications for the pathogenesis of EBV-positive lymphomas in man. J Exp Med. 1991 Jan 1;173(1):147–158. doi: 10.1084/jem.173.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]





