Abstract
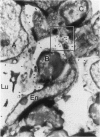
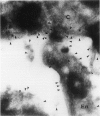
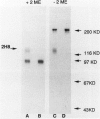
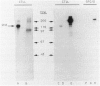
Human CD31 is a recently characterized molecule present on leukocytes, platelets, and endothelium. Its function is not known. Because it is a member of the immunoglobulin superfamily and structurally homologous to carcinoembryonic antigen, a putative intercellular adhesion molecule, it is believed that CD31 may function also as an adhesion molecule. In this report, we characterize the cellular reactivity of a monoclonal antibody to a murine protein that is homologous to CD31. To delineate the cellular reactivity of the murine CD31 homologue recognized by our monoclonal antibody, we used immunoperoxidase and immunoelectron microscopic techniques. The most striking finding was that the putative murine homolog of CD31 is expressed in particularly high amounts on endothelium-adherent lymphocytes transmigrating across sinusoidal or venular vascular boundaries. Such a distribution was apparent in draining murine lymph nodes during the peak of an immune response after immunization with a protein antigen in adjuvant, a situation in which there are many transmigrating lymphocytes. Immunoelectron microscopic analysis also shows that CD31 is predominantly distributed on portions of transmigrating lymphocytes that are in contact with or adjacent to areas of contact with endothelial cells. These findings suggest a previously undescribed role for CD31 in lymphocyte recruitment and transmigration.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Muller W. A., Buck C. A., Newman P. J. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991 Sep;114(5):1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda S. M., Oliver P. D., Romer L. H., Buck C. A. EndoCAM: a novel endothelial cell-cell adhesion molecule. J Cell Biol. 1990 Apr;110(4):1227–1237. doi: 10.1083/jcb.110.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen S. A., Weinberg D. S., Abbas A. K. Histologic analysis of T lymphocyte activation in reactive lymph nodes. J Immunol. 1991 Sep 1;147(5):1537–1541. [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Hamann A., Jablonski-Westrich D., Duijvestijn A., Butcher E. C., Baisch H., Harder R., Thiele H. G. Evidence for an accessory role of LFA-1 in lymphocyte-high endothelium interaction during homing. J Immunol. 1988 Feb 1;140(3):693–699. [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Lesley J., Hyman R., Schulte R., Trotter J. Expression of transferrin receptor on murine hematopoietic progenitors. Cell Immunol. 1984 Jan;83(1):14–25. doi: 10.1016/0008-8749(84)90220-x. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Muller W. A., Ratti C. M., McDonnell S. L., Cohn Z. A. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989 Aug 1;170(2):399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P. J., Berndt M. C., Gorski J., White G. C., 2nd, Lyman S., Paddock C., Muller W. A. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990 Mar 9;247(4947):1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Ohto H., Maeda H., Shibata Y., Chen R. F., Ozaki Y., Higashihara M., Takeuchi A., Tohyama H. A novel leukocyte differentiation antigen: two monoclonal antibodies TM2 and TM3 define a 120-kd molecule present on neutrophils, monocytes, platelets, and activated lymphoblasts. Blood. 1985 Oct;66(4):873–881. [PubMed] [Google Scholar]
- Simmons D. L., Walker C., Power C., Pigott R. Molecular cloning of CD31, a putative intercellular adhesion molecule closely related to carcinoembryonic antigen. J Exp Med. 1990 Jun 1;171(6):2147–2152. doi: 10.1084/jem.171.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S. J., Daukas G., Zigmond S. H. Asymmetric distribution of the chemotactic peptide receptor on polymorphonuclear leukocytes. J Cell Biol. 1984 Oct;99(4 Pt 1):1461–1467. doi: 10.1083/jcb.99.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T., Singer S. J. Improved procedures for immunoferritin labeling of ultrathin frozen sections. J Cell Biol. 1976 Dec;71(3):894–906. doi: 10.1083/jcb.71.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torimoto Y., Rothstein D. M., Dang N. H., Schlossman S. F., Morimoto C. CD31, a novel cell surface marker for CD4 cells of suppressor lineage, unaltered by state of activation. J Immunol. 1992 Jan 15;148(2):388–396. [PubMed] [Google Scholar]
- van Mourik J. A., Leeksma O. C., Reinders J. H., de Groot P. G., Zandbergen-Spaargaren J. Vascular endothelial cells synthesize a plasma membrane protein indistinguishable from the platelet membrane glycoprotein IIa. J Biol Chem. 1985 Sep 15;260(20):11300–11306. [PubMed] [Google Scholar]