Abstract
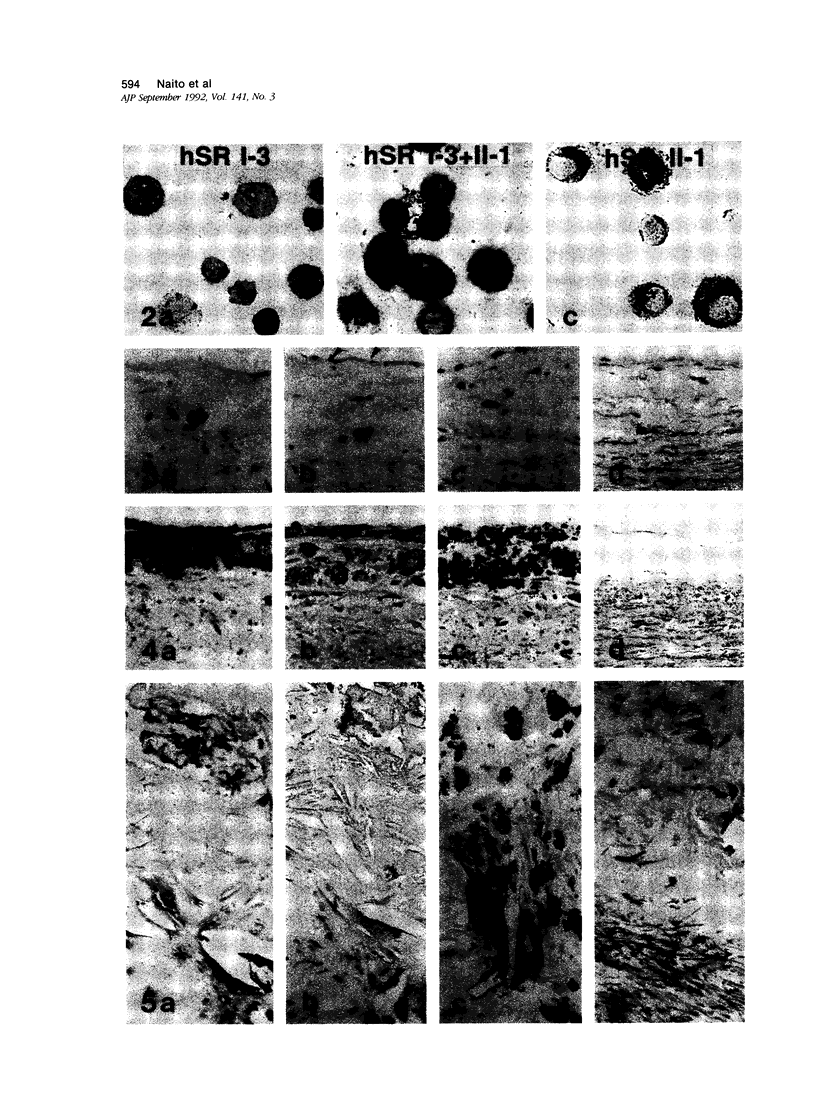
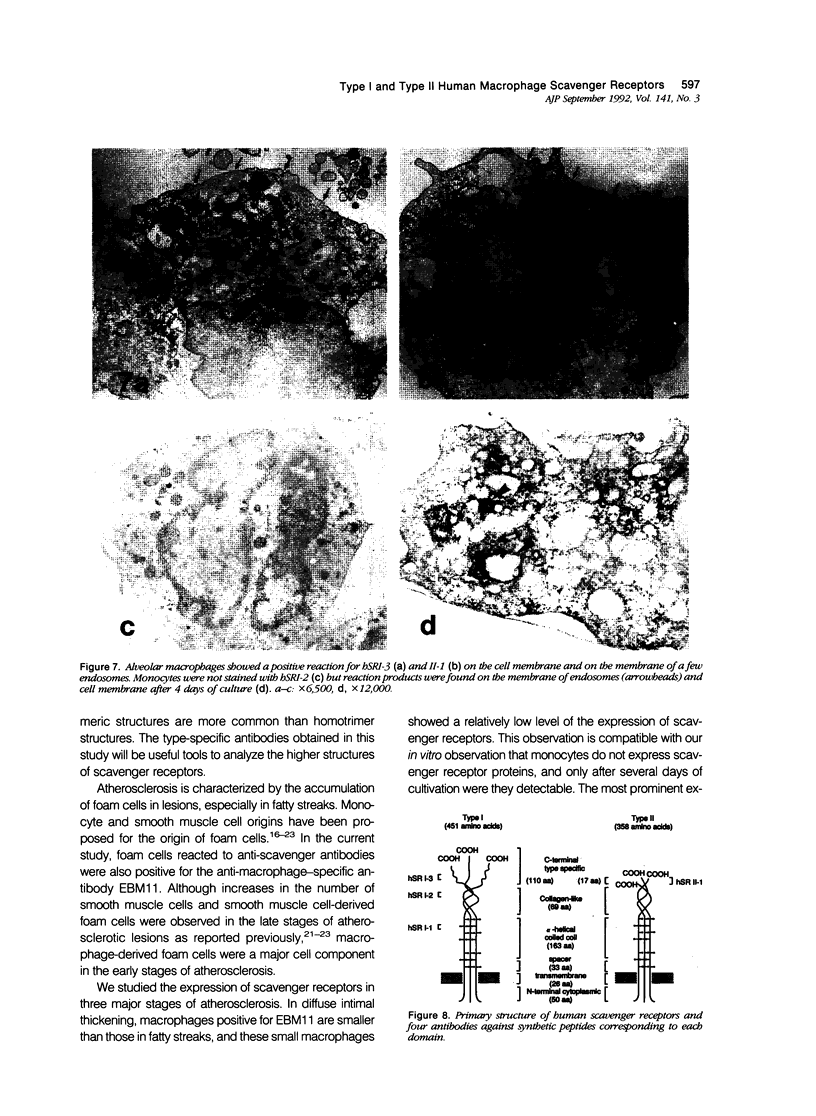
Macrophage scavenger receptors are trimeric membrane glycoproteins implicated in the pathologic deposition of cholesterol in arterial walls during atherogenesis. Two types of cDNAs for functional human receptors have been cloned, but their physiologic roles remain obscure. To study the expression of these receptors, the authors generated antibodies against scavenger receptor type-specific synthetic peptide. Immunohistochemical examination using these antibodies and other anti-human receptor antibodies shows that type I and type II receptor proteins can be detected in foam cells in various stages of atherosclerosis, most evidently in fatty streaks. Co-expression of the two types of receptor protein was also detected in macrophages of various organs. Both types of the protein were detected on the surface and the membrane of endosomes in macrophages. These results indicate that both type I and type II scavenger receptors are expressed and functionally active in physiologic and pathologic conditions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. S., Goldstein J. L. Atherosclerosis. Scavenging for receptors. Nature. 1990 Feb 8;343(6258):508–509. doi: 10.1038/343508a0. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R. Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis. 1984 Jul-Aug;4(4):341–356. doi: 10.1161/01.atv.4.4.341. [DOI] [PubMed] [Google Scholar]
- Freeman M., Ashkenas J., Rees D. J., Kingsley D. M., Copeland N. G., Jenkins N. A., Krieger M. An ancient, highly conserved family of cysteine-rich protein domains revealed by cloning type I and type II murine macrophage scavenger receptors. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8810–8814. doi: 10.1073/pnas.87.22.8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981 May;103(2):191–200. [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome W. G., Lewis J. C. Early atherogenesis in White Carneau pigeons. I. Leukocyte margination and endothelial alterations at the celiac bifurcation. Am J Pathol. 1984 Jul;116(1):56–68. [PMC free article] [PubMed] [Google Scholar]
- Joris I., Zand T., Nunnari J. J., Krolikowski F. J., Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol. 1983 Dec;113(3):341–358. [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Freeman M., Rohrer L., Zabrecky J., Matsudaira P., Krieger M. Type I macrophage scavenger receptor contains alpha-helical and collagen-like coiled coils. Nature. 1990 Feb 8;343(6258):531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- Kodama T., Reddy P., Kishimoto C., Krieger M. Purification and characterization of a bovine acetyl low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9238–9242. doi: 10.1073/pnas.85.23.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Naito M., Itakura H., Ikemoto S., Asaoka H., Hayakawa I., Kanamori H., Aburatani H., Takaku F., Suzuki H. Human macrophage scavenger receptors: primary structure, expression, and localization in atherosclerotic lesions. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9133–9137. doi: 10.1073/pnas.87.23.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito M., Kodama T., Matsumoto A., Doi T., Takahashi K. Tissue distribution, intracellular localization, and in vitro expression of bovine macrophage scavenger receptors. Am J Pathol. 1991 Dec;139(6):1411–1423. [PMC free article] [PubMed] [Google Scholar]
- Pitas R. E., Boyles J., Mahley R. W., Bissell D. M. Uptake of chemically modified low density lipoproteins in vivo is mediated by specific endothelial cells. J Cell Biol. 1985 Jan;100(1):103–117. doi: 10.1083/jcb.100.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer L., Freeman M., Kodama T., Penman M., Krieger M. Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature. 1990 Feb 8;343(6258):570–572. doi: 10.1038/343570a0. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Rosenfeld M., Ross R., Gown A. M. Immunocytochemical analysis of cellular components in atherosclerotic lesions. Use of monoclonal antibodies with the Watanabe and fat-fed rabbit. Arteriosclerosis. 1986 Nov-Dec;6(6):601–613. doi: 10.1161/01.atv.6.6.601. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Hirata M., Yoshikawa Y., Nagafuchi Y., Toyoshima H., Watanabe T. Role of macrophages in atherosclerosis. Sequential observations of cholesterol-induced rabbit aortic lesion by the immunoperoxidase technique using monoclonal antimacrophage antibody. Lab Invest. 1985 Jul;53(1):80–90. [PubMed] [Google Scholar]
- van Furth R. Origin and turnover of monocytes and macrophages. Curr Top Pathol. 1989;79:125–150. [PubMed] [Google Scholar]