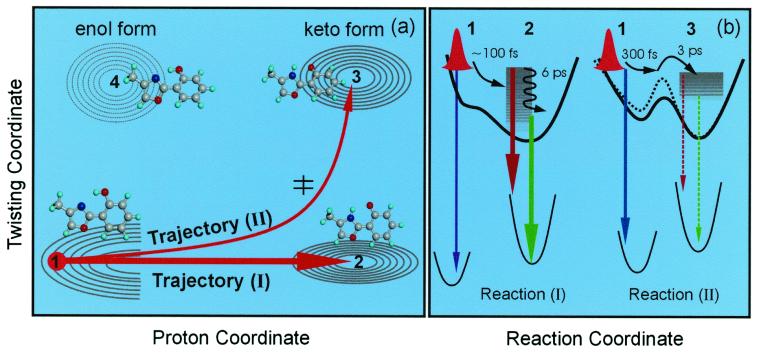
Figure 5.
(a) The potential energy contour map for two of the nuclear coordinates involved: a schematic for two trajectories of the direct proton-transfer (I) and the one involving twisting (II). The initial wave packet (structure 1) bifurcates and evolves as shown. The dominant channel mainly involves the proton transfer coordinate (N⋅⋅⋅H—O) to form the keto form (structure 2). The minor channel initially follows the proton motion and finally proceeds along the twisting motion of the two heterocyclic rings reaching the keto rotamer minimum (structure 3). The corresponding molecular structures also are shown. (b) Potential energy curves along the reaction coordinate for the two trajectories with the observed emission indicated schematically. The black dashed line represents the potential energy in the protein environment, and the barrier is increased because of the rigid protein–ligand binding structure. Note that rotamers of nonplanar enol ground state (near structure 1) in this picture can undergo proton transfer on the upper surface after an initial twist toward planarity.